SNTF immunostaining reveals previously undetected axonal pathology in traumatic brain injury
- PMID: 26589592
- PMCID: PMC4780426
- DOI: 10.1007/s00401-015-1506-0
SNTF immunostaining reveals previously undetected axonal pathology in traumatic brain injury
Abstract
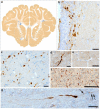
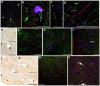
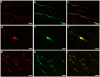
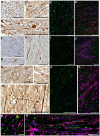
Diffuse axonal injury (DAI) is a common feature of severe traumatic brain injury (TBI) and may also be a predominant pathology in mild TBI or "concussion". The rapid deformation of white matter at the instant of trauma can lead to mechanical failure and calcium-dependent proteolysis of the axonal cytoskeleton in association with axonal transport interruption. Recently, a proteolytic fragment of alpha-II spectrin, "SNTF", was detected in serum acutely following mild TBI in patients and was prognostic for poor clinical outcome. However, direct evidence that this fragment is a marker of DAI has yet to be demonstrated in either humans following TBI or in models of mild TBI. Here, we used immunohistochemistry (IHC) to examine for SNTF in brain tissue following both severe and mild TBI. Human severe TBI cases (survival <7d; n = 18) were compared to age-matched controls (n = 16) from the Glasgow TBI archive. We also examined brains from an established model of mild TBI at 6, 48 and 72 h post-injury versus shams. IHC specific for SNTF was compared to that of amyloid precursor protein (APP), the current standard for DAI diagnosis, and other known markers of axonal pathology including non-phosphorylated neurofilament-H (SMI-32), neurofilament-68 (NF-68) and compacted neurofilament-medium (RMO-14) using double and triple immunofluorescent labeling. Supporting its use as a biomarker of DAI, SNTF immunoreactive axons were observed at all time points following both human severe TBI and in the model of mild TBI. Interestingly, SNTF revealed a subpopulation of degenerating axons, undetected by the gold-standard marker of transport interruption, APP. While there was greater axonal co-localization between SNTF and APP after severe TBI in humans, a subset of SNTF positive axons displayed no APP accumulation. Notably, some co-localization was observed between SNTF and the less abundant neurofilament subtype markers. Other SNTF positive axons, however, did not co-localize with any other markers. Similarly, RMO-14 and NF-68 positive axonal pathology existed independent of SNTF and APP. These data demonstrate that multiple pathological axonal phenotypes exist post-TBI and provide insight into a more comprehensive approach to the neuropathological assessment of DAI.
Keywords: Amyloid precursor protein; Axonal pathology; Concussion; Diffuse axonal injury; Mild TBI; Neurofilaments; SNTF; Spectrin breakdown; TBI; Traumatic brain injury.
Figures




References
-
- Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. - PubMed
-
- Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–563. - PubMed
-
- Baines AJ. Evolution of spectrin function in cytoskeletal and membrane networks. Biochemical Society transactions. 2009;37:796–803. - PubMed
-
- Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma. 2007;24:1447–1459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

