Trichomonas vaginalis Lipophosphoglycan Exploits Binding to Galectin-1 and -3 to Modulate Epithelial Immunity
- PMID: 26589797
- PMCID: PMC4705417
- DOI: 10.1074/jbc.M115.651497
Trichomonas vaginalis Lipophosphoglycan Exploits Binding to Galectin-1 and -3 to Modulate Epithelial Immunity
Abstract
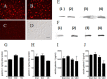
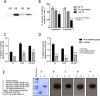
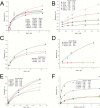
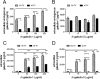
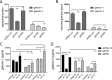
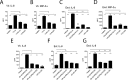
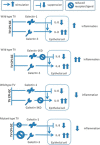
Trichomoniasis is the most common non-viral sexually transmitted infection caused by the vaginotropic extracellular protozoan parasite Trichomonas vaginalis. The infection is recurrent, with no lasting immunity, often asymptomatic, and linked to pregnancy complications and risk of viral infection. The molecular mechanisms of immune evasion by the parasite are poorly understood. We demonstrate that galectin-1 and -3 are expressed by the human cervical and vaginal epithelial cells and act as pathogen-recognition receptors for the ceramide phosphoinositol glycan core (CPI-GC) of the dominant surface protozoan lipophosphoglycan (LPG). We used an in vitro model with siRNA galectin knockdown epithelial clones, recombinant galectins, clinical Trichomonas isolates, and mutant protozoan derivatives to dissect the function of galectin-1 and -3 in the context of Trichomonas infection. Galectin-1 suppressed chemokines that facilitate recruitment of phagocytes, which can eliminate extracellular protozoa (IL-8) or bridge innate to adaptive immunity (MIP-3α and RANTES (regulated on activation normal T cell expressed and secreted)). Silencing galectin-1 increased and adding exogenous galectin-1 suppressed chemokine responses to Trichomonas or CPI-GC/LPG. In contrast, silencing galectin-3 reduced IL-8 response to LPG. Live Trichomonas depleted the extracellular levels of galectin-3. Clinical isolates and mutant Trichomonas CPI-GC that had reduced affinity to galectin-3 but maintained affinity to galectin-1 suppressed chemokine expression. Thus via CPI-GC binding, Trichomonas is capable of regulating galectin bioavailability and function to the benefit of its parasitic survival. These findings suggest novel approaches to control trichomoniasis and warrant further studies of galectin-binding diversity among clinical isolates as a possible source for symptom disparity in parasitic infections.
Keywords: CCL-20 (MIP-3α); CCL5 (RANTES); Interleukin 8 (IL-8); cytokine; galectin; human vagina; inflammation; interleukin; parasite; sexually transmitted infection.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis.Cell Microbiol. 2008 Oct;10(10):2078-90. doi: 10.1111/j.1462-5822.2008.01190.x. Epub 2008 Jul 10. Cell Microbiol. 2008. PMID: 18637021 Free PMC article.
-
The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert.Sex Transm Infect. 2013 Sep;89(6):460-6. doi: 10.1136/sextrans-2013-051052. Epub 2013 Jul 31. Sex Transm Infect. 2013. PMID: 23903808 Free PMC article.
-
Structural details and composition of Trichomonas vaginalis lipophosphoglycan in relevance to the epithelial immune function.Glycoconj J. 2009 Jan;26(1):3-17. doi: 10.1007/s10719-008-9157-1. Epub 2008 Jul 6. Glycoconj J. 2009. PMID: 18604640 Free PMC article.
-
Impact of T. vaginalis infection on innate immune responses and reproductive outcome.J Reprod Immunol. 2009 Dec;83(1-2):185-9. doi: 10.1016/j.jri.2009.08.007. Epub 2009 Oct 21. J Reprod Immunol. 2009. PMID: 19850356 Free PMC article. Review.
-
The Role of Purinergic Signaling in Trichomonas vaginalis Infection.Curr Top Med Chem. 2021;21(3):181-192. doi: 10.2174/1568026620999200904122212. Curr Top Med Chem. 2021. PMID: 32888270 Review.
Cited by
-
Seminal Plasma Glycoproteins as Potential Ligands of Lectins Engaged in Immunity Regulation.Int J Environ Res Public Health. 2022 Aug 23;19(17):10489. doi: 10.3390/ijerph191710489. Int J Environ Res Public Health. 2022. PMID: 36078205 Free PMC article. Review.
-
Trichomonas vaginalis Induces NLRP3 Inflammasome Activation and Pyroptotic Cell Death in Human Macrophages.J Innate Immun. 2019;11(1):86-98. doi: 10.1159/000493585. Epub 2018 Nov 2. J Innate Immun. 2019. PMID: 30391945 Free PMC article.
-
Galectins - Important players of the immune response to CNS parasitic infection.Brain Behav Immun Health. 2021 Feb 17;13:100221. doi: 10.1016/j.bbih.2021.100221. eCollection 2021 May. Brain Behav Immun Health. 2021. PMID: 34589740 Free PMC article. Review.
-
Galectin-1 Ameliorates Influenza A H1N1pdm09 Virus-Induced Acute Lung Injury.Front Microbiol. 2020 Jun 12;11:1293. doi: 10.3389/fmicb.2020.01293. eCollection 2020. Front Microbiol. 2020. PMID: 32595629 Free PMC article.
-
The L-Rhamnose Biosynthetic Pathway in Trichomonas vaginalis: Identification and Characterization of UDP-D-Glucose 4,6-dehydratase.Int J Mol Sci. 2022 Nov 23;23(23):14587. doi: 10.3390/ijms232314587. Int J Mol Sci. 2022. PMID: 36498914 Free PMC article.
References
-
- World Health Organization (2012) Global incidence and prevalence of selected curable sexually transmitted infections-2008. World Health Organization, Geneva, Switzerland, http://apps.who.int/iris/bitstream/10665/75181/1/9789241503839_eng.pdf
-
- Nyame A. K., Kawar Z. S., and Cummings R. D. (2004) Antigenic glycans in parasitic infections: implications for vaccines and diagnostics. Arch. Biochem. Biophys. 426, 182–200 - PubMed
-
- Turco S. J., and Descoteaux A. (1992) The lipophosphoglycan of Leishmania parasites. Annu. Rev. Microbiol. 46, 65–94 - PubMed
-
- Ferguson M. A. (1999) The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J. Cell Sci. 112, 2799–2809 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

