WASp-dependent actin cytoskeleton stability at the dendritic cell immunological synapse is required for extensive, functional T cell contacts
- PMID: 26590149
- PMCID: PMC5404712
- DOI: 10.1189/jlb.2A0215-050RR
WASp-dependent actin cytoskeleton stability at the dendritic cell immunological synapse is required for extensive, functional T cell contacts
Abstract
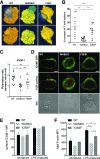
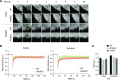
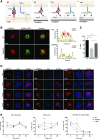
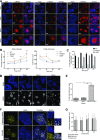
The immunological synapse is a highly structured and molecularly dynamic interface between communicating immune cells. Although the immunological synapse promotes T cell activation by dendritic cells, the specific organization of the immunological synapse on the dendritic cell side in response to T cell engagement is largely unknown. In this study, confocal and electron microscopy techniques were used to investigate the role of dendritic cell actin regulation in immunological synapse formation, stabilization, and function. In the dendritic cell-restricted absence of the Wiskott-Aldrich syndrome protein, an important regulator of the actin cytoskeleton in hematopoietic cells, the immunological synapse contact with T cells occupied a significantly reduced surface area. At a molecular level, the actin network localized to the immunological synapse exhibited reduced stability, in particular, of the actin-related protein-2/3-dependent, short-filament network. This was associated with decreased polarization of dendritic cell-associated ICAM-1 and MHC class II, which was partially dependent on Wiskott-Aldrich syndrome protein phosphorylation. With the use of supported planar lipid bilayers incorporating anti-ICAM-1 and anti-MHC class II antibodies, the dendritic cell actin cytoskeleton organized into recognizable synaptic structures but interestingly, formed Wiskott-Aldrich syndrome protein-dependent podosomes within this area. These findings demonstrate that intrinsic dendritic cell cytoskeletal remodeling is a key regulatory component of normal immunological synapse formation, likely through consolidation of adhesive interaction and modulation of immunological synapse stability.
Keywords: Arp2/3; DC; FRAP; ICAM-1; podosomes.
© The Author(s).
Figures
References
-
- Bunnell S. C., Kapoor V., Trible R. P., Zhang W., Samelson L. E. (2001) Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity 14, 315–329. - PubMed
-
- Al-Alwan M. M., Rowden G., Lee T. D., West K. A. (2001) The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J. Immunol. 166, 1452–1456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

