Modular Representation of Luminance Polarity in the Superficial Layers of Primary Visual Cortex
- PMID: 26590348
- PMCID: PMC4753785
- DOI: 10.1016/j.neuron.2015.10.019
Modular Representation of Luminance Polarity in the Superficial Layers of Primary Visual Cortex
Abstract
The spatial arrangement of luminance increments (ON) and decrements (OFF) falling on the retina provides a wealth of information used by central visual pathways to construct coherent representations of visual scenes. But how the polarity of luminance change is represented in the activity of cortical circuits remains unclear. Using wide-field epifluorescence and two-photon imaging we demonstrate a robust modular representation of luminance polarity (ON or OFF) in the superficial layers of ferret primary visual cortex. Polarity-specific domains are found with both uniform changes in luminance and single light/dark edges, and include neurons selective for orientation and direction of motion. The integration of orientation and polarity preference is evident in the selectivity and discrimination capabilities of most layer 2/3 neurons. We conclude that polarity selectivity is an integral feature of layer 2/3 neurons, ensuring that the distinction between light and dark stimuli is available for further processing in downstream extrastriate areas.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


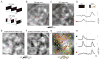



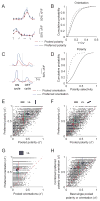
Comment in
-
A Division of Light and Dark in the Visual Cortex.Neuron. 2015 Nov 18;88(4):624-6. doi: 10.1016/j.neuron.2015.11.006. Neuron. 2015. PMID: 26590338
References
-
- Alonso JM, Martinez LM. Functional connectivity between simple cells and complex cells in cat striate cortex. Nat Neurosci. 1998;1:395–403. - PubMed
-
- Bartlett JR, Doty RW., Sr Response of units in striate cortex of squirrel monkeys to visual and electrical stimuli. J Neurophysiol. 1974;37:621–641. - PubMed
-
- Blasdel GG, Salama G. Voltage-sensitive dyes reveal a modular organization in monkey striate cortex. Nature. 1986;321:579–585. - PubMed
-
- Boyd JD, Matsubara JA. Laminar and columnar patterns of geniculocortical projections in the cat: relationship to cytochrome oxidase. The Journal of comparative neurology. 1996;365:659–682. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

