Descending Command Neurons in the Brainstem that Halt Locomotion
- PMID: 26590422
- PMCID: PMC4899047
- DOI: 10.1016/j.cell.2015.10.074
Descending Command Neurons in the Brainstem that Halt Locomotion
Abstract
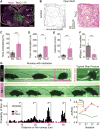
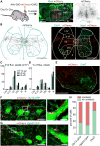
The episodic nature of locomotion is thought to be controlled by descending inputs from the brainstem. Most studies have largely attributed this control to initiating excitatory signals, but little is known about putative commands that may specifically determine locomotor offset. To link identifiable brainstem populations to a potential locomotor stop signal, we used developmental genetics and considered a discrete neuronal population in the reticular formation: the V2a neurons. We find that those neurons constitute a major excitatory pathway to locomotor areas of the ventral spinal cord. Selective activation of V2a neurons of the rostral medulla stops ongoing locomotor activity, owing to an inhibition of premotor locomotor networks in the spinal cord. Moreover, inactivation of such neurons decreases spontaneous stopping in vivo. Therefore, the V2a "stop neurons" represent a glutamatergic descending pathway that favors immobility and may thus help control the episodic nature of locomotion.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
We declare no conflict of interest regarding this work.
Figures





References
-
- Al-Mosawie A, Wilson JM, Brownstone RM. Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur J Neurosci. 2007;26:3003–3015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

