Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target
- PMID: 26590448
- PMCID: PMC5408507
- DOI: 10.1053/j.ajkd.2015.09.027
Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target
Abstract
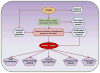
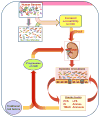
Also known as the "second human genome," the gut microbiome plays important roles in both the maintenance of health and the pathogenesis of disease. The symbiotic relationship between host and microbiome is disturbed due to the proliferation of dysbiotic bacteria in patients with chronic kidney disease (CKD). Fermentation of protein and amino acids by gut bacteria generates excess amounts of potentially toxic compounds such as ammonia, amines, thiols, phenols, and indoles, but the generation of short-chain fatty acids is reduced. Impaired intestinal barrier function in patients with CKD permits translocation of gut-derived uremic toxins into the systemic circulation, contributing to the progression of CKD, cardiovascular disease, insulin resistance, and protein-energy wasting. The field of microbiome research is still nascent, but is evolving rapidly. Establishing symbiosis to treat uremic syndrome is a novel concept, but if proved effective, it will have a significant impact on the management of patients with CKD.
Keywords: Gut microbiome; amine; ammonia; chronic kidney disease (CKD); end-stage renal disease (ESRD); indole; metabolome; microbial metabolite; p-cresyl sulfate (PCS); phenol; review; thiol; urea; uremic syndrome; uremic toxin.
Copyright © 2016 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. - PubMed
-
- Lederberg J. Infectious history. Science. 2000;288(5464):287–293. - PubMed
-
- Hawrelak JA, Myers SP. The causes of intestinal dysbiosis: a review. Altern Med Rev. 2004;9(2):180–197. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

