Raloxifene as an Adjunctive Treatment for Postmenopausal Women With Schizophrenia: A 24-Week Double-Blind, Randomized, Parallel, Placebo-Controlled Trial
- PMID: 26591005
- PMCID: PMC4753610
- DOI: 10.1093/schbul/sbv149
Raloxifene as an Adjunctive Treatment for Postmenopausal Women With Schizophrenia: A 24-Week Double-Blind, Randomized, Parallel, Placebo-Controlled Trial
Abstract
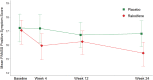
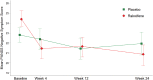
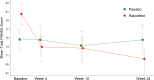
The potential therapeutic utility of estrogens in schizophrenia is increasingly being recognized. Raloxifene, a selective estrogen receptor modulator, appears to act similarly to estrogens on dopamine and serotonin brain systems. One previous trial by our team found that raloxifene was useful to improve negative, positive, and general psychopathological symptoms, without having the negative side effects of estrogens. In this study, we assess the utility of raloxifene in treating negative and other psychotic symptoms in postmenopausal women with schizophrenia exhibiting prominent negative symptoms. This was a 24-week, randomized, parallel, double-blind, placebo-controlled study. Patients were recruited from the inpatient and outpatient departments of Parc Sanitari Sant Joan de Déu, Hospital Universitari Institut Pere Mata, and Corporació Sanitària Parc Taulí. Seventy postmenopausal women with schizophrenia (DSM-IV) were randomized to either adjunctive raloxifene (38 women) or adjunctive placebo (32 women). Psychopathological symptoms were assessed at baseline and at weeks 4, 12, and 24 with the Positive and Negative Syndrome Scale (PANSS) and the Scale for the Assessment of Negative Symptoms (SANS). The addition of raloxifene (60 mg/d) to regular antipsychotic treatment significantly reduced negative (P = .027), general (P = .003), and total symptomatology (P = .005) measured with the PANSS during the 24-week trial, as compared to women receiving placebo. Also Alogia SANSS subscale improved more in the raloxifene (P = .048) than the placebo group. In conclusion, raloxifene improved negative and general psychopathological symptoms, compared with antipsychotic medication alone, in postmenopausal women with schizophrenia. These data replicate our previous results with a larger sample and a longer follow-up.
Trial registration: NCT01573637.
Keywords: SERM; estrogen; negative symptoms.
© The Author 2015. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand. 2000;401:3–38. - PubMed
-
- Usall J, Haro JM, Ochoa S, Márquez M, Araya S; Needs of Patients with Schizophrenia group. Influence of gender on social outcome in schizophrenia. Acta Psychiatr Scand. 2002;106:337–342. - PubMed
-
- Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull. 1990;16:185–194. - PubMed
-
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

