Aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1 activation and DC-STAMP expression
- PMID: 26592521
- PMCID: PMC4753374
- DOI: 10.1038/aps.2015.85
Aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1 activation and DC-STAMP expression
Abstract
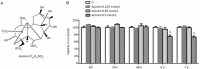
Aim: Aconiti Lateralis Radix Preparata is a traditional Chinese medicine used to treat chronic arthritis and is highly effective against rheumatoid arthritis. However, the effects of aconine, a derivative of aconitum alkaloids, on osteoclasts, which can absorb bone, remain unknown. Here, we investigated the effects of aconine on osteoclast differentiation and bone resorption in vitro.
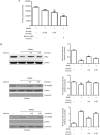
Methods: The viability of mouse leukemic monocyte/macrophage cell line RAW264.7 was measured using CCK-8 assays. Osteoclast differentiation was induced by incubation of RAW264.7 cells in the presence of RANKL, and assessed with TRAP staining assay. Bone resorption was examined with bone resorption pits assay. The expression of relevant genes and proteins was analyzed using RT-PCR and Western blots. The activation of NF-κB and nuclear factor of activated T-cells (NFAT) was examined using stable NF-κB and NFATc1 luciferase reporter gene systems, RT-PCR and Western blot analysis.
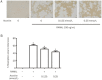
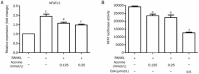
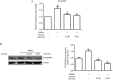
Results: Aconine (0.125, 0.25 μmol/L) did not affect the viability of RAW264.7 cells, but dose-dependently inhibited RANKL-induced osteoclast formation and bone resorptive activity. Furthermore, aconine dose-dependently inhibited the RANKL-induced activation of NF-κB and NFATc1 in RAW264.7 cells, and subsequently reduced the expression of osteoclast-specific genes (c-Src, β3-Integrin, cathepsin K and MMP-9) and the expression of dendritic cell-specific transmembrane protein (DC-STAMP), which played an important role in cell-cell fusion.
Conclusion: These findings suggest that aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing the activation of NF-κB and NFATc1 and the expression of the cell-cell fusion molecule DC-STAMP.
Figures

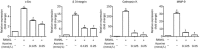
References
-
- 1Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003; 423: 337–42. - PubMed
-
- 3Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol 2006; 24: 33–63. - PubMed
-
- 4Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 2007; 7: 292–304. - PubMed
-
- 5Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev 2009; 231: 241–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

