Behavioral and neurophysiological evidence that lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the acquisition and extinction of fear learning
- PMID: 26593151
- PMCID: PMC4718746
- DOI: 10.1016/j.nlm.2015.11.006
Behavioral and neurophysiological evidence that lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the acquisition and extinction of fear learning
Abstract
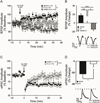
The lateral/basolateral amygdala (BLA) is crucial to the acquisition and extinction of Pavlovian fear conditioning, and synaptic plasticity in this region is considered to be a neural correlate of learned fear. We recently reported that activation of BLA β3-adrenoreceptors (β3-ARs) selectively enhances lateral paracapsular (LPC) feed-forward GABAergic inhibition onto BLA pyramidal neurons, and that intra-BLA infusion of a β3-AR agonist reduces measures of unconditioned anxiety-like behavior. Here, we utilized a combination of behavioral and electrophysiological approaches to characterize the role of BLA LPCs in the acquisition of fear and extinction learning in adult male Long-Evans rats. We report that intra-BLA microinjection of β3-AR agonists (BRL37344 or SR58611A, 1μg/0.5μL/side) prior to training fear conditioning or extinction blocks the expression of these behaviors 24h later. Furthermore,ex vivo low-frequency stimulation of the external capsule (LFS; 1Hz, 15min), which engages LPC synapses, induces LTP of BLA fEPSPs, while application of a β3-AR agonist (SR58611A, 5μM) induces LTD of fEPSPs when combined with LFS. Interestingly, fEPSP LTP is not observed in recordings from fear conditioned animals, suggesting that fear learning may engage the same mechanisms that induce synaptic plasticity at this input. In support of this, we find that LFS produces LTD of inhibitory postsynaptic currents (iLTD) at LPC GABAergic synapses, and that this effect is also absent following fear conditioning. Taken together, these data provide preliminary evidence that modulation of LPC GABAergic synapses can influence the acquisition and extinction of fear learning and related synaptic plasticity in the BLA.
Keywords: Fear-potentiated startle; Inhibitory plasticity; Noradrenaline.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
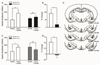
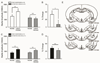
References
-
- Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nature neuroscience. 2003;6:587–592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

