Functional Dynamics within the Human Ribosome Regulate the Rate of Active Protein Synthesis
- PMID: 26593721
- PMCID: PMC4660248
- DOI: 10.1016/j.molcel.2015.09.013
Functional Dynamics within the Human Ribosome Regulate the Rate of Active Protein Synthesis
Abstract
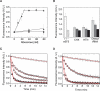
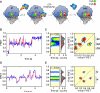
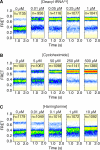
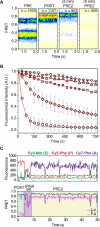
The regulation of protein synthesis contributes to gene expression in both normal physiology and disease, yet kinetic investigations of the human translation mechanism are currently lacking. Using single-molecule fluorescence imaging methods, we have quantified the nature and timing of structural processes in human ribosomes during single-turnover and processive translation reactions. These measurements reveal that functional complexes exhibit dynamic behaviors and thermodynamic stabilities distinct from those observed for bacterial systems. Structurally defined sub-states of pre- and post-translocation complexes were sensitive to specific inhibitors of the eukaryotic ribosome, demonstrating the utility of this platform to probe drug mechanism. The application of three-color single-molecule fluorescence resonance energy transfer (smFRET) methods further revealed a long-distance allosteric coupling between distal tRNA binding sites within ribosomes bearing three tRNAs, which contributed to the rate of processive translation.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
A new view of protein synthesis: mapping the free energy landscape of the ribosome using single-molecule FRET.Biopolymers. 2008 Jul;89(7):565-77. doi: 10.1002/bip.20961. Biopolymers. 2008. PMID: 18286627 Free PMC article. Review.
-
Transfer RNA-mediated regulation of ribosome dynamics during protein synthesis.Nat Struct Mol Biol. 2011 Aug 21;18(9):1043-51. doi: 10.1038/nsmb.2098. Nat Struct Mol Biol. 2011. PMID: 21857664 Free PMC article.
-
Single-molecule observations of ribosome function.Curr Opin Struct Biol. 2009 Feb;19(1):103-9. doi: 10.1016/j.sbi.2009.01.002. Epub 2009 Feb 14. Curr Opin Struct Biol. 2009. PMID: 19223173 Free PMC article. Review.
-
The ribosome uses cooperative conformational changes to maximize and regulate the efficiency of translation.Proc Natl Acad Sci U S A. 2014 Aug 19;111(33):12073-8. doi: 10.1073/pnas.1401864111. Epub 2014 Aug 1. Proc Natl Acad Sci U S A. 2014. PMID: 25085895 Free PMC article.
-
Single-molecule fluorescence measurements of ribosomal translocation dynamics.Mol Cell. 2011 May 6;42(3):367-77. doi: 10.1016/j.molcel.2011.03.024. Mol Cell. 2011. PMID: 21549313 Free PMC article.
Cited by
-
RNA Dynamics by NMR Spectroscopy.Chembiochem. 2019 Nov 4;20(21):2685-2710. doi: 10.1002/cbic.201900072. Epub 2019 Jul 17. Chembiochem. 2019. PMID: 30997719 Free PMC article. Review.
-
Human Protein Synthesis Requires aminoacyl-tRNA Pivoting During Proofreading.bioRxiv [Preprint]. 2025 Jun 26:2025.06.23.661150. doi: 10.1101/2025.06.23.661150. bioRxiv. 2025. PMID: 40667029 Free PMC article. Preprint.
-
A small ribosome-associated ncRNA globally inhibits translation by restricting ribosome dynamics.RNA Biol. 2021 Dec;18(12):2617-2632. doi: 10.1080/15476286.2021.1935573. Epub 2021 Jun 13. RNA Biol. 2021. PMID: 34121604 Free PMC article.
-
Dynamics of IRES-mediated translation.Philos Trans R Soc Lond B Biol Sci. 2017 Mar 19;372(1716):20160177. doi: 10.1098/rstb.2016.0177. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28138065 Free PMC article. Review.
-
Impacts of ribosomal RNA sequence variation on gene expression and phenotype.Philos Trans R Soc Lond B Biol Sci. 2025 Mar 6;380(1921):20230379. doi: 10.1098/rstb.2023.0379. Epub 2025 Mar 6. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40045785 Free PMC article. Review.
References
-
- Andersen CBF, Becker T, Blau M, Anand M, Halic M, Balar B, Mielke T, Boesen T, Pedersen JS, Spahn CMT, et al. Structure of eEF3 and the mechanism of transfer RNA release from the E-site. Nature. 2006;443:663–668. - PubMed
-
- Anger AM, Armache J-P, Berninghausen O, Habeck M, Subklewe M, Wilson DN, Beckmann R. Structures of the human and Drosophila 80S ribosome. Nature. 2013;497:80–85. - PubMed
-
- Blaha G, Nierhaus KH. Features and functions of the ribosomal E site. Cold Spring Harb. Symp. Quant. Biol. 2001;66:135–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

