A Critical Role for Cysteine 57 in the Biological Functions of Selenium Binding Protein-1
- PMID: 26593911
- PMCID: PMC4661901
- DOI: 10.3390/ijms161126043
A Critical Role for Cysteine 57 in the Biological Functions of Selenium Binding Protein-1
Abstract
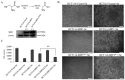
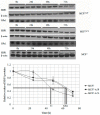
The concentration of selenium-binding protein1 (SBP1) is often lower in tumors than in the corresponding tissue and lower levels have been associated with poor clinical outcomes. SBP1 binds tightly selenium although what role selenium plays in its biological functions remains unknown. Previous studies indicated that cysteine 57 is the most likely candidate amino acid for selenium binding. In order to investigate the role of cysteine 57 in SBP1, this amino acid was altered to a glycine and the mutated protein was expressed in human cancer cells. The SBP1 half-life, as well as the cellular response to selenite cytotoxicity, was altered by this change. The ectopic expression of SBP1(GLY) also caused mitochondrial damage in HCT116 cells. Taken together, these results indicated that cysteine 57 is a critical determinant of SBP1 function and may play a significant role in mitochondrial function.
Keywords: glutathione peroxidase 1; mitochondrial damage; selenite cytotoxicity; selenium-binding protein1.
Figures



Similar articles
-
Selenium-Binding Protein 1 in Human Health and Disease.Int J Mol Sci. 2018 Nov 2;19(11):3437. doi: 10.3390/ijms19113437. Int J Mol Sci. 2018. PMID: 30400135 Free PMC article. Review.
-
Reduction of selenium-binding protein 1 sensitizes cancer cells to selenite via elevating extracellular glutathione: a novel mechanism of cancer-specific cytotoxicity of selenite.Free Radic Biol Med. 2015 Feb;79:186-96. doi: 10.1016/j.freeradbiomed.2014.11.015. Epub 2014 Nov 29. Free Radic Biol Med. 2015. PMID: 25445402
-
Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein.Carcinogenesis. 2010 Aug;31(8):1360-6. doi: 10.1093/carcin/bgq114. Epub 2010 Jun 7. Carcinogenesis. 2010. PMID: 20530237 Free PMC article.
-
Molecular cross-talk between members of distinct families of selenium containing proteins.Mol Nutr Food Res. 2014 Jan;58(1):117-23. doi: 10.1002/mnfr.201300543. Epub 2013 Nov 13. Mol Nutr Food Res. 2014. PMID: 24395536 Free PMC article. Review.
-
Selenium binding protein 1 protects renal tubular epithelial cells from ferroptosis by upregulating glutathione peroxidase 4.Chem Biol Interact. 2024 Apr 25;393:110944. doi: 10.1016/j.cbi.2024.110944. Epub 2024 Mar 20. Chem Biol Interact. 2024. PMID: 38518851
Cited by
-
A Caenorhabditis elegans ortholog of human selenium-binding protein 1 is a pro-aging factor protecting against selenite toxicity.Redox Biol. 2020 Jan;28:101323. doi: 10.1016/j.redox.2019.101323. Epub 2019 Sep 11. Redox Biol. 2020. PMID: 31557719 Free PMC article.
-
SBP1 promotes tumorigenesis of thyroid cancer through TXN/NIS pathway.Mol Med. 2023 Sep 8;29(1):121. doi: 10.1186/s10020-023-00700-y. Mol Med. 2023. PMID: 37684566 Free PMC article.
-
Selenium-binding protein 1 alters energy metabolism in prostate cancer cells.Prostate. 2020 Sep;80(12):962-976. doi: 10.1002/pros.24028. Epub 2020 Jun 8. Prostate. 2020. PMID: 32511787 Free PMC article.
-
Selenium-Binding Protein 1 Indicates Myocardial Stress and Risk for Adverse Outcome in Cardiac Surgery.Nutrients. 2019 Aug 25;11(9):2005. doi: 10.3390/nu11092005. Nutrients. 2019. PMID: 31450690 Free PMC article.
-
Selenium-Binding Protein 1 in Human Health and Disease.Int J Mol Sci. 2018 Nov 2;19(11):3437. doi: 10.3390/ijms19113437. Int J Mol Sci. 2018. PMID: 30400135 Free PMC article. Review.
References
-
- Huang C., Ding G., Gu C., Zhou J., Kuang M., Ji Y., He Y., Kondo T., Fan J. Decreased selenium-binding protein 1 enhances glutathione peroxidase 1 activity and downregulates HIF-1α to promote hepatocellular carcinoma invasiveness. Clin. Cancer Res. 2012;18:3042–3053. doi: 10.1158/1078-0432.CCR-12-0183. - DOI - PubMed
-
- Raucci R., Colonna G., Guerriero E., Capone F., Accardo M., Castello G., Costantini S. Structural and functional studies of the human selenium binding protein-1 and its involvement in hepatocellular carcinoma. Biochim. Biophys. Acta. 2011;1814:513–522. doi: 10.1016/j.bbapap.2011.02.006. - DOI - PubMed
-
- Fang W., Goldberg M.L., Pohl N.M., Bi X., Tong C., Xiong B., Koh T.J., Diamond A.M., Yang W. Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis. 2010;31:1360–1366. doi: 10.1093/carcin/bgq114. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

