Probing the Lipid Annular Belt by Gas-Phase Dissociation of Membrane Proteins in Nanodiscs
- PMID: 26594028
- PMCID: PMC4736441
- DOI: 10.1002/anie.201508289
Probing the Lipid Annular Belt by Gas-Phase Dissociation of Membrane Proteins in Nanodiscs
Abstract
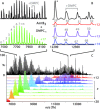
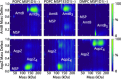
Interactions between membrane proteins and lipids are often crucial for structure and function yet difficult to define because of their dynamic and heterogeneous nature. Here, we use mass spectrometry to demonstrate that membrane protein oligomers ejected from nanodiscs in the gas phase retain large numbers of lipid interactions. The complex mass spectra that result from gas-phase dissociation were assigned using a Bayesian deconvolution algorithm together with mass defect analysis, allowing us to count individual lipid molecules bound to membrane proteins. Comparison of the lipid distributions measured by mass spectrometry with molecular dynamics simulations reveals that the distributions correspond to distinct lipid shells that vary according to the type of protein-lipid interactions. Our results demonstrate that nanodiscs offer the potential for native mass spectrometry to probe interactions between membrane proteins and the wider lipid environment.
Keywords: lipid annulus; mass spectrometry; membrane proteins; nanodiscs; protein-lipid interactions.
© 2015 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

