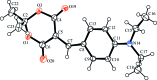
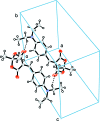
Crystal structure of 5-[4-(di-ethyl-amino)-benzyl-idene]-2,2-dimethyl-1,3-dioxane-4,6-dione
- PMID: 26594416
- PMCID: PMC4647437
- DOI: 10.1107/S2056989015017673
Crystal structure of 5-[4-(di-ethyl-amino)-benzyl-idene]-2,2-dimethyl-1,3-dioxane-4,6-dione
Abstract
The title compound, C17H21NO4, consists of substituted Meldrum's acid with a [4-(di-ethyl-amino)-phen-yl]methyl-idene fragment attached to the fifth position. The heterocycle assumes a distorted boat conformation. The planar part of heterocycle is almost coplanar with the benzene ring due to the presence of a long conjugated system in the mol-ecule. This leads to the formation of C-H⋯O-type intra-molecular contacts. As a result of the absence of hydrogen-bond donors in the structure, the crystal packing is controlled by van der Waals forces and weak C-H⋯O inter-actions, which associate the mol-ecules into inversion dimers.
Keywords: 5-arylmethylene-2,2-dimethyl-1,3-dioxan-4,6-dione; arylidene Meldrum’s acid; crystal structure; intramolecular hydrogen bonding; organic synthesis.
Figures
References
-
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., Giacovazzo, C., Mallamo, M., Mazzone, A., Polidori, G. & Spagna, R. (2012). J. Appl. Cryst. 45, 357–361.
-
- Crawford, L. A. & McNab, H. (2009). Collect. Czech. Chem. Commun. 74, 995–1009.
-
- Dey, T., Ghosh, S., Ghosh, S. & Mukherjee, A. K. (2015). J. Mol. Struct. 1092, 51–62.
-
- El Maatougui, A., JhonnyAzuaje, B. S. P., Coelho, A., Cano, E., Yanez, M., Lopez, C., Yaziji, V., Carbajales, C. & Sotelo, E. (2012). Combin. Chem. High Throughput Screen. 15, 551–554. - PubMed
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
LinkOut - more resources
Full Text Sources
Research Materials