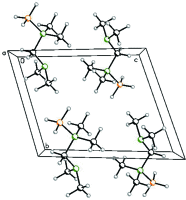
Crystal structure of borated N,N,N',N'-tetra-methyldi-amino-methane
- PMID: 26594453
- PMCID: PMC4647418
- DOI: 10.1107/S2056989015016813
Crystal structure of borated N,N,N',N'-tetra-methyldi-amino-methane
Abstract
In the title compound, {[(di-methyl-amino)-meth-yl]di-methyl-amine}-trihydridoboron, C5H17BN2, the tetra-hedral geometry of the N-C-N unit is slightly disorted. As a result of the bulky amine substituents, a wider N-C-N angle of 113.6 (1)° is observed. The bond lengths between the N atom and methyl groups are slighly elongated to 1.481 (2) and 1.482 (2) Å at the borated N atom, whereas the distances between the other N atom and its methyl groups are only 1.461 (2) and 1.462 (2) Å. The studied crystal was twinned. The twin data refinement was subsequently carried out with a scale factor of 0.263 (1). The two lattices of the twin domains were rotated by 179.84°.
Keywords: amine; borane; crystal structure; twin.
Figures
References
-
- Agilent (2014). CrysAlis PRO. Agilent Technologies, Yarnton, England.
-
- Bera, B., Patil, Y. P., Nethaji, M. & Jagirdar, B. R. (2011). Dalton Trans. 40, 10592–10597. - PubMed
-
- Burg, A. B. & Schlesinger, H. I. (1937). J. Am. Chem. Soc. 59, 780–787.
-
- Chitsaz, S., Breyhan, T., Pauls, J. & Neumüller, B. (2002). Z. Anorg. Allg. Chem. 628, 956–964.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
LinkOut - more resources
Full Text Sources
Research Materials