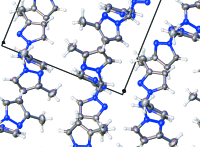
Crystal structure of tris-(3-methyl-1H-pyrazol-1-yl)methane
- PMID: 26594542
- PMCID: PMC4645007
- DOI: 10.1107/S2056989015017247
Crystal structure of tris-(3-methyl-1H-pyrazol-1-yl)methane
Abstract
The title mol-ecule, C13H16N6, crystallizes from hexane as a mol-ecular crystal with no strong inter-molecular inter-actions (the shortest C-H⋯N contact is longer than 3.38 Å). A relatively short intra-molecular contact (3.09 Å) has a C-H⋯N angle of 118° which is quite small to be still considered a hydrogen bond. The three pyrazole rings form a propeller-like motif, with one methylpyrazole unit almost perpendicular to the mean plane of the three rings [82.20 (6)°]. The other two methylpyrazole units, with nitrogen donor atoms oriented in opposite directions, are oriented at 67.26 (6) and 72.53 (6)° to the mean plane.
Keywords: 1,1′,1′′-methanetriyltris(3-methyl-1H-pyrazole); crystal structure; tripyrazolylmethane.
Figures
References
-
- Bruker (2013). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2014). SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
-
- Goodman, M. A., Nazarenko, A. Y., Casavant, B. J., Li, Z., Brennessel, W. W., DeMarco, M. J., Long, G. & Goodman, M. S. (2012). Inorg. Chem. 51, 1084–1093. - PubMed
-
- Jameson, D. L. & Castellano, R. K. (1998). Inorg. Synth 32, 51–63.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases