Hepatitis B Virus Reactivation and Prophylaxis During Solid Tumor Chemotherapy: A Systematic Review and Meta-analysis
- PMID: 26595058
- PMCID: PMC6410701
- DOI: 10.7326/M15-1121
Hepatitis B Virus Reactivation and Prophylaxis During Solid Tumor Chemotherapy: A Systematic Review and Meta-analysis
Abstract
Background: Solid tumor chemotherapy regimens pose a risk for hepatitis B virus (HBV) reactivation, but screening and antiviral prophylaxis remains controversial because of insufficient evidence.
Purpose: To determine the risk for HBV reactivation with and without antiviral prophylaxis and the effectiveness of prophylaxis in adults with solid tumors and chronic or resolved HBV infection.
Data sources: MEDLINE through 1 July 2015 and Web of Science, Cochrane Central Register of Controlled Trials, TOXNET, and Scopus through 1 March 2015.
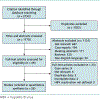
Study selection: 26 English-language observational studies and randomized, controlled trials in patients with chronic or resolved HBV receiving chemotherapy for solid tumors.
Data extraction: Study characteristics, quality, and risk of bias were assessed by 1 researcher and verified by another independent researcher.
Data synthesis: Random-effects model meta-analyses were used to estimate the risk and odds ratio (OR) of reactivation with versus without antiviral prophylaxis. Reactivation in chronic HBV without prophylaxis ranged from 4% to 68% (median, 25%) with substantial heterogeneity. Prophylaxis reduced the risk for HBV reactivation (OR, 0.12 [95% CI, 0.06 to 0.22]), HBV-related hepatitis (OR, 0.18 [CI, 0.10 to 0.32]), and chemotherapy interruption (OR, 0.10 [CI, 0.04 to 0.27]). In 3 studies of patients with resolved HBV infection, none received HBV prophylaxis and reactivation risk ranged from 0.3% to 9.0%.
Limitations: Significant heterogeneity in underlying study populations and treatment regimens, incomplete baseline data, possibility of publication bias, and limited study quality. Most studies were observational and from Asia.
Conclusion: In patients with chronic HBV receiving solid tumor chemotherapy, the risk for HBV reactivation is similar to the risk with other types of immunosuppressive therapy. Results support HBV screening and antiviral prophylaxis before initiation of chemotherapy for solid tumors.
Primary funding source: National Center for Advancing Translational Sciences and National Institutes of Health.
Conflict of interest statement
Disclosures: Dr. Paul reports grants from National Center for Advancing Translational Sciences, National Institutes of Health, and the 2013 to 2014 Bristol-Myers Squibb Virology Research Training Program during the conduct of the study. Dr. Wong reports nonfinancial support from the American Association for the Study of Liver Diseases (as a member of systematic review writing group for an HBV practice guideline) outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewedat
Figures






References
-
- Alter MJ. Epidemiology of hepatitis B in Europe and worldwide. J Hepatol. 2003;39 Suppl 1:S64–9. [PMID: ] - PubMed
-
- Wasley A, Kruszon-Moran D, Kuhnert W, Simard EP, Finelli L, McQuillan G, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. [PMID: ] - PubMed
-
- Shouval D, Shibolet O. Immunosuppression and HBV reactivation. Semin Liver Dis. 2013;33:167–77. [PMID: ] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
