Conserved molecular interactions in centriole-to-centrosome conversion
- PMID: 26595382
- PMCID: PMC4719191
- DOI: 10.1038/ncb3274
Conserved molecular interactions in centriole-to-centrosome conversion
Abstract
Centrioles are required to assemble centrosomes for cell division and cilia for motility and signalling. New centrioles assemble perpendicularly to pre-existing ones in G1-S and elongate throughout S and G2. Fully elongated daughter centrioles are converted into centrosomes during mitosis to be able to duplicate and organize pericentriolar material in the next cell cycle. Here we show that centriole-to-centrosome conversion requires sequential loading of Cep135, Ana1 (Cep295) and Asterless (Cep152) onto daughter centrioles during mitotic progression in both Drosophila melanogaster and human. This generates a molecular network spanning from the inner- to outermost parts of the centriole. Ana1 forms a molecular strut within the network, and its essential role can be substituted by an engineered fragment providing an alternative linkage between Asterless and Cep135. This conserved architectural framework is essential for loading Asterless or Cep152, the partner of the master regulator of centriole duplication, Plk4. Our study thus uncovers the molecular basis for centriole-to-centrosome conversion that renders daughter centrioles competent for motherhood.
Figures


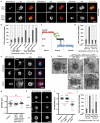
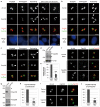
Comment in
-
Cytoskeleton: Centriole maturation to motherhood.Nat Rev Mol Cell Biol. 2016 Jan;17(1):4. doi: 10.1038/nrm.2015.12. Epub 2015 Dec 9. Nat Rev Mol Cell Biol. 2016. PMID: 26648266 No abstract available.
Similar articles
-
Cdk1 Phosphorylates Drosophila Sas-4 to Recruit Polo to Daughter Centrioles and Convert Them to Centrosomes.Dev Cell. 2016 Jun 20;37(6):545-57. doi: 10.1016/j.devcel.2016.05.022. Dev Cell. 2016. PMID: 27326932 Free PMC article.
-
Asterless is a scaffold for the onset of centriole assembly.Nature. 2010 Oct 7;467(7316):714-8. doi: 10.1038/nature09445. Epub 2010 Sep 19. Nature. 2010. PMID: 20852615
-
Ana1 helps recruit Polo to centrioles to promote mitotic PCM assembly and centriole elongation.J Cell Sci. 2021 Jul 15;134(14):jcs258987. doi: 10.1242/jcs.258987. Epub 2021 Jul 22. J Cell Sci. 2021. PMID: 34156068 Free PMC article.
-
Show me your license, please: deregulation of centriole duplication mechanisms that promote amplification.Cell Mol Life Sci. 2013 Mar;70(6):1021-34. doi: 10.1007/s00018-012-1102-6. Epub 2012 Aug 15. Cell Mol Life Sci. 2013. PMID: 22892665 Free PMC article. Review.
-
Role of Polo-like Kinases Plk1 and Plk4 in the Initiation of Centriole Duplication-Impact on Cancer.Cells. 2022 Feb 24;11(5):786. doi: 10.3390/cells11050786. Cells. 2022. PMID: 35269408 Free PMC article. Review.
Cited by
-
How the newborn centriole becomes a mother.Cell Cycle. 2016 Jun 17;15(12):1521-2. doi: 10.1080/15384101.2016.1164566. Epub 2016 May 25. Cell Cycle. 2016. PMID: 27223270 Free PMC article. No abstract available.
-
The SKP1-Cullin-F-box E3 ligase βTrCP and CDK2 cooperate to control STIL abundance and centriole number.Open Biol. 2018 Feb;8(2):170253. doi: 10.1098/rsob.170253. Open Biol. 2018. PMID: 29445034 Free PMC article.
-
How centrioles acquire the ability to reproduce.Elife. 2017 Mar 8;6:e25358. doi: 10.7554/eLife.25358. Elife. 2017. PMID: 28271993 Free PMC article.
-
Cytoskeleton: Centriole maturation to motherhood.Nat Rev Mol Cell Biol. 2016 Jan;17(1):4. doi: 10.1038/nrm.2015.12. Epub 2015 Dec 9. Nat Rev Mol Cell Biol. 2016. PMID: 26648266 No abstract available.
-
Once and only once: mechanisms of centriole duplication and their deregulation in disease.Nat Rev Mol Cell Biol. 2018 May;19(5):297-312. doi: 10.1038/nrm.2017.127. Epub 2018 Jan 24. Nat Rev Mol Cell Biol. 2018. PMID: 29363672 Free PMC article. Review.
References
-
- Sillibourne JE, et al. Assessing the localization of centrosomal proteins by PALM/STORM nanoscopy. Cytoskeleton. 2011;68:619–627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

