Taxanes enhance trastuzumab-mediated ADCC on tumor cells through NKG2D-mediated NK cell recognition
- PMID: 26595802
- PMCID: PMC4807996
- DOI: 10.18632/oncotarget.6353
Taxanes enhance trastuzumab-mediated ADCC on tumor cells through NKG2D-mediated NK cell recognition
Abstract
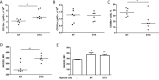
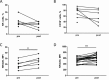
Recent clinical data indicate a synergistic therapeutic effect between trastuzumab and taxanes in neoadjuvantly treated HER2-positive breast cancer (BC) patients. In HER2+ BC experimental models and patients, we investigated whether this synergy depends on the ability of drug-induced stress to improve NK cell effectiveness and thus trastuzumab-mediated ADCC. HER2+ BC cell lines BT474 and MDAMB361 treated with docetaxel showed up-modulation of NK activator ligands both in vitro and in vivo, accompanied by a 15-40% increase in in vitro trastuzumab-mediated ADCC; antibodies blocking the NKG2D receptor significantly reduced this enhancement. NKG2D receptor expression was increased by docetaxel treatment in circulating and splenic NK cells from mice xenografted with tumor cells, an increase related to expansion of the CD11b+Ly6G+ cell population. Accordingly, NK cells derived from HER2+ BC patients after treatment with taxane-containing therapy expressed higher levels of NKG2D receptor than before treatment. Moreover, plasma obtained from these patients recapitulated the modulation of NKG2D on healthy donors' NK cells, improving their trastuzumab-mediated activity in vitro. This enhancement occurred mainly using plasma from patients with low NKG2D basal expression. Our results indicate that taxanes increase tumor susceptibility to ADCC by acting on tumor and NK cells, and suggest that taxanes concomitantly administered with trastuzumab could maximize the antibody effect, especially in patients with low basal immune effector cytotoxic activity.
Keywords: ADCC; NK cell; NKG2D; breast cancer; docetaxel.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
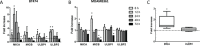



Similar articles
-
Lenalidomide enhances antibody-dependent cellular cytotoxicity of solid tumor cells in vitro: influence of host immune and tumor markers.Cancer Immunol Immunother. 2011 Jan;60(1):61-73. doi: 10.1007/s00262-010-0919-9. Epub 2010 Sep 17. Cancer Immunol Immunother. 2011. PMID: 20848094 Free PMC article.
-
Fc-optimized NKG2D-Fc constructs induce NK cell antibody-dependent cellular cytotoxicity against breast cancer cells independently of HER2/neu expression status.J Immunol. 2014 Oct 15;193(8):4261-72. doi: 10.4049/jimmunol.1400872. Epub 2014 Sep 12. J Immunol. 2014. PMID: 25217158
-
Expression of the ULBP ligands for NKG2D by B-NHL cells plays an important role in determining their susceptibility to rituximab-induced ADCC.Int J Cancer. 2009 Jul 1;125(1):212-21. doi: 10.1002/ijc.24351. Int J Cancer. 2009. PMID: 19358282
-
Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications.Scand J Immunol. 2013 Aug;78(2):120-9. doi: 10.1111/sji.12072. Scand J Immunol. 2013. PMID: 23679194 Review.
-
Natural killer group 2D receptor and its ligands in cancer immune escape.Mol Cancer. 2019 Feb 27;18(1):29. doi: 10.1186/s12943-019-0956-8. Mol Cancer. 2019. PMID: 30813924 Free PMC article. Review.
Cited by
-
Heterogeneity of colon cancer: from bench to bedside.ESMO Open. 2017 Aug 22;2(3):e000218. doi: 10.1136/esmoopen-2017-000218. eCollection 2017. ESMO Open. 2017. PMID: 29209524 Free PMC article. Review.
-
Effect of anti-CD4 mAb induced by inhibiting B cell disorder on immune reconstruction of HIV-infected immunological non-responders.Mol Med. 2025 Jun 20;31(1):244. doi: 10.1186/s10020-025-01286-3. Mol Med. 2025. PMID: 40542350 Free PMC article.
-
Early immune modulation by single-agent trastuzumab as a marker of trastuzumab benefit.Br J Cancer. 2018 Dec;119(12):1487-1494. doi: 10.1038/s41416-018-0318-0. Epub 2018 Nov 27. Br J Cancer. 2018. PMID: 30478407 Free PMC article.
-
Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer.Cancers (Basel). 2018 Sep 20;10(10):342. doi: 10.3390/cancers10100342. Cancers (Basel). 2018. PMID: 30241301 Free PMC article. Review.
-
Predicting the Efficacy of HER2-Targeted Therapies: A Look at the Host.Dis Markers. 2017;2017:7849108. doi: 10.1155/2017/7849108. Epub 2017 Dec 18. Dis Markers. 2017. PMID: 29403144 Free PMC article. Review.
References
-
- Madarnas Y, Trudeau M, Franek JA, McCready D, Pritchard KI, Messersmith H. Adjuvant/neoadjuvant trastuzumab therapy in women with HER-2/neu-overexpressing breast cancer: a systematic review. Cancer Treat Rev. 2008;34:539–557. - PubMed
-
- Bullock K, Blackwell K. Clinical efficacy of taxane-trastuzumab combination regimens for HER-2-positive metastatic breast cancer. Oncologist. 2008;13:515–525. - PubMed
-
- Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, Pusztai L, Green MC, Singletary SE, Hunt KK, Sahin AA, Esteva F, Symmans WF, Ewer MS, Buchholz TA, Hortobagyi GN. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

