PML-RARA requires DNA methyltransferase 3A to initiate acute promyelocytic leukemia
- PMID: 26595813
- PMCID: PMC4701540
- DOI: 10.1172/JCI82897
PML-RARA requires DNA methyltransferase 3A to initiate acute promyelocytic leukemia
Abstract
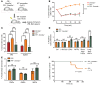
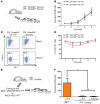
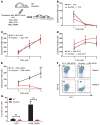
The DNA methyltransferases DNMT3A and DNMT3B are primarily responsible for de novo methylation of specific cytosine residues in CpG dinucleotides during mammalian development. While loss-of-function mutations in DNMT3A are highly recurrent in acute myeloid leukemia (AML), DNMT3A mutations are almost never found in AML patients with translocations that create oncogenic fusion genes such as PML-RARA, RUNX1-RUNX1T1, and MLL-AF9. Here, we explored how DNMT3A is involved in the function of these fusion genes. We used retroviral vectors to express PML-RARA, RUNX1-RUNX1T1, or MLL-AF9 in bone marrow cells derived from WT or DNMT3A-deficient mice. Additionally, we examined the phenotypes of hematopoietic cells from Ctsg-PML-RARA mice, which express PML-RARA in early hematopoietic progenitors and myeloid precursors, with or without DNMT3A. We determined that the methyltransferase activity of DNMT3A, but not DNMT3B, is required for aberrant PML-RARA-driven self-renewal ex vivo and that DNMT3A is dispensable for RUNX1-RUNX1T1- and MLL-AF9-driven self-renewal. Furthermore, both the PML-RARA-driven competitive transplantation advantage and development of acute promyelocytic leukemia (APL) required DNMT3A. Together, these findings suggest that PML-RARA requires DNMT3A to initiate APL in mice.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- K12 CA167540/CA/NCI NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- P30 CA91842/CA/NCI NIH HHS/United States
- CA162086/CA/NCI NIH HHS/United States
- CA101937/CA/NCI NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- P01 CA101937/CA/NCI NIH HHS/United States
- R35 CA197561/CA/NCI NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- R01 CA162086/CA/NCI NIH HHS/United States
- CA083962/CA/NCI NIH HHS/United States
- R01 CA083962/CA/NCI NIH HHS/United States
- CA197561/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

