Targeted cellular ablation based on the morphology of malignant cells
- PMID: 26596248
- PMCID: PMC4657158
- DOI: 10.1038/srep17157
Targeted cellular ablation based on the morphology of malignant cells
Abstract
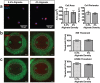
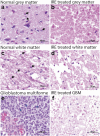
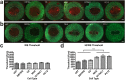
Treatment of glioblastoma multiforme (GBM) is especially challenging due to a shortage of methods to preferentially target diffuse infiltrative cells, and therapy-resistant glioma stem cell populations. Here we report a physical treatment method based on electrical disruption of cells, whose action depends strongly on cellular morphology. Interestingly, numerical modeling suggests that while outer lipid bilayer disruption induced by long pulses (~100 μs) is enhanced for larger cells, short pulses (~1 μs) preferentially result in high fields within the cell interior, which scale in magnitude with nucleus size. Because enlarged nuclei represent a reliable indicator of malignancy, this suggested a means of preferentially targeting malignant cells. While we demonstrate killing of both normal and malignant cells using pulsed electric fields (PEFs) to treat spontaneous canine GBM, we proposed that properly tuned PEFs might provide targeted ablation based on nuclear size. Using 3D hydrogel models of normal and malignant brain tissues, which permit high-resolution interrogation during treatment testing, we confirmed that PEFs could be tuned to preferentially kill cancerous cells. Finally, we estimated the nuclear envelope electric potential disruption needed for cell death from PEFs. Our results may be useful in safely targeting the therapy-resistant cell niches that cause recurrence of GBM tumors.
Conflict of interest statement
R.V.D and M.B.S are inventors on pending and issued patents related to the work. The authors declare no other competing financial interest.
Figures






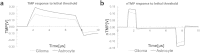
References
-
- Siu A. et al. Radiation necrosis following treatment of high grade glioma—a review of the literature and current understanding. Acta Neurochirurgica 154, 191–201 (2012). - PubMed
-
- Crossen J. R., Garwood D., Glatstein E. & Neuwelt E. A. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol 12, 627–642 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

