Stable isotope method to measure drug release from nanomedicines
- PMID: 26596375
- PMCID: PMC4688069
- DOI: 10.1016/j.jconrel.2015.10.042
Stable isotope method to measure drug release from nanomedicines
Abstract
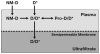
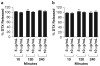
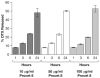
Existing methods to measure nanomedicine drug release in biological matrices are inadequate. A novel drug release method utilizing a stable isotope tracer has been developed. Stable isotope-labeled drug is spiked into plasma containing nanomedicine. The labeled drug equilibrates with plasma components identical to the normoisotopic drug released from the nanomedicine formulation. Therefore, the ultrafilterable fraction of the isotope-labeled drug represents a reliable measure of free normoisotopic drug fraction in plasma, and can be used to calculate nanomedicine encapsulated and unencapsulated drug fractions. To demonstrate the utility of this method, we performed a plasma drug release study with both a fast releasing commercial docetaxel formulation, Taxotere®, and a delayed releasing nanomicellar formulation of a docetaxel prodrug, Procet 8. The instability of the unencapsulated prodrug in plasma allowed us to compare our calculated prodrug release and docetaxel conversion with the actual docetaxel concentration measured directly without fractionation. Drug release estimates for the fast releasing Taxotere formulation demonstrated accuracy deviation and precision (%CV) of <15%. For the controlled release Procet 8 formulation, we calculated a slow release and conversion of the prodrug in rat plasma that was highly correlated with the direct docetaxel measurement (R(2)=0.98). We believe that this method will have tremendous utility in the development and regulatory evaluation of nanomedicines, and aid in determination of generic bioequivalence.
Keywords: Drug release method; Fractionation in biological matrix; Nanomedicine; Nanotechnology; Stable isotope dilution.
Copyright © 2015. Published by Elsevier B.V.
Figures




Comment in
-
Novel approach to measure drug release from nanomedicines.J Control Release. 2015 Dec 28;220(Pt A):568. doi: 10.1016/j.jconrel.2015.11.026. J Control Release. 2015. PMID: 26686299 No abstract available.
Similar articles
-
Distinguishing Pharmacokinetics of Marketed Nanomedicine Formulations Using a Stable Isotope Tracer Assay.ACS Pharmacol Transl Sci. 2020 Mar 13;3(3):547-558. doi: 10.1021/acsptsci.0c00011. eCollection 2020 Jun 12. ACS Pharmacol Transl Sci. 2020. PMID: 32566919 Free PMC article.
-
Ultrafiltration Drug Release Assay Utilizing a Stable Isotope Tracer: Version 1.0.2017 Apr. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method PHA-2. 2017 Apr. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method PHA-2. PMID: 39013065 Free Books & Documents. Review.
-
Prediction of nanoparticle prodrug metabolism by pharmacokinetic modeling of biliary excretion.J Control Release. 2013 Dec 10;172(2):558-67. doi: 10.1016/j.jconrel.2013.04.025. Epub 2013 May 9. J Control Release. 2013. PMID: 23664969 Free PMC article.
-
Improved Ultrafiltration Method to Measure Drug Release from Nanomedicines Utilizing a Stable Isotope Tracer.Methods Mol Biol. 2018;1682:223-239. doi: 10.1007/978-1-4939-7352-1_19. Methods Mol Biol. 2018. PMID: 29039106
-
Nanomedicine: enhancement of chemotherapeutical efficacy of docetaxel by using a biodegradable nanoparticle formulation.Curr Pharm Des. 2010 Jul;16(21):2308-20. doi: 10.2174/138161210791920487. Curr Pharm Des. 2010. PMID: 20618152 Review.
Cited by
-
Methodological needs in the quality and safety characterisation of nanotechnology-based health products: Priorities for method development and standardisation.J Control Release. 2021 Aug 10;336:192-206. doi: 10.1016/j.jconrel.2021.06.016. Epub 2021 Jun 12. J Control Release. 2021. PMID: 34126169 Free PMC article. Review.
-
Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval.Adv Drug Deliv Rev. 2020;156:80-118. doi: 10.1016/j.addr.2020.09.009. Epub 2020 Sep 24. Adv Drug Deliv Rev. 2020. PMID: 32980449 Free PMC article. Review.
-
Industrialization's eye view on theranostic nanomedicine.Front Chem. 2022 Aug 19;10:918715. doi: 10.3389/fchem.2022.918715. eCollection 2022. Front Chem. 2022. PMID: 36059870 Free PMC article. Review.
-
Distinguishing Pharmacokinetics of Marketed Nanomedicine Formulations Using a Stable Isotope Tracer Assay.ACS Pharmacol Transl Sci. 2020 Mar 13;3(3):547-558. doi: 10.1021/acsptsci.0c00011. eCollection 2020 Jun 12. ACS Pharmacol Transl Sci. 2020. PMID: 32566919 Free PMC article.
-
Entry of nanoparticles into cells and tissues: status and challenges.Beilstein J Nanotechnol. 2024 Aug 12;15:1017-1029. doi: 10.3762/bjnano.15.83. eCollection 2024. Beilstein J Nanotechnol. 2024. PMID: 39161463 Free PMC article.
References
-
- Zolnik BS, Stern ST, Kaiser JM, Heakal Y, Clogston JD, Kester M, McNeil SE. Rapid distribution of liposomal short-chain ceramide in vitro and in vivo. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:1709–1715. - PubMed
-
- Ambardekar VV, Stern ST. NBCD Pharmacokinetics and Drug Release Methods. In: Crommelin DJA, de Vlieger JSB, editors. Non-Biological Complex Drugs; The Science and the Regulatory Landscape. Springer International Publishing; 2015. pp. 261–287.
-
- Thies RL, Cowens DW, Cullis PR, Bally MB, Mayer LD. Method for rapid separation of liposome-associated doxorubicin from free doxorubicin in plasma. Analytical biochemistry. 1990;188:65–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

