Neomycin Sulfate Improves the Antimicrobial Activity of Mupirocin-Based Antibacterial Ointments
- PMID: 26596945
- PMCID: PMC4750660
- DOI: 10.1128/AAC.02083-15
Neomycin Sulfate Improves the Antimicrobial Activity of Mupirocin-Based Antibacterial Ointments
Abstract
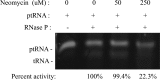
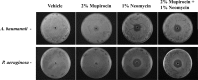
In the midst of the current antimicrobial pipeline void, alternative approaches are needed to reduce the incidence of infection and decrease reliance on last-resort antibiotics for the therapeutic intervention of bacterial pathogens. In that regard, mupirocin ointment-based decolonization and wound maintenance practices have proven effective in reducing Staphylococcus aureus transmission and mitigating invasive disease. However, the emergence of mupirocin-resistant strains has compromised the agent's efficacy, necessitating new strategies for the prevention of staphylococcal infections. Herein, we set out to improve the performance of mupirocin-based ointments. A screen of a Food and Drug Administration (FDA)-approved drug library revealed that the antibiotic neomycin sulfate potentiates the antimicrobial activity of mupirocin, whereas other library antibiotics did not. Preliminary mechanism of action studies indicate that neomycin's potentiating activity may be mediated by inhibition of the organism's RNase P function, an enzyme that is believed to participate in the tRNA processing pathway immediately upstream of the primary target of mupirocin. The improved antimicrobial activity of neomycin and mupirocin was maintained in ointment formulations and reduced S. aureus bacterial burden in murine models of nasal colonization and wound site infections. Combination therapy improved upon the effects of either agent alone and was effective in the treatment of contemporary methicillin-susceptible, methicillin-resistant, and high-level mupirocin-resistant S. aureus strains. From these perspectives, combination mupirocin-and-neomycin ointments appear to be superior to that of mupirocin alone and warrant further development.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Clearance of nasal Staphylococcus aureus colonization with triple antibiotic ointment.J Drugs Dermatol. 2012 Dec;11(12):1490-2. J Drugs Dermatol. 2012. PMID: 23377521 Clinical Trial.
-
Contemporary antimicrobial activity of triple antibiotic ointment: a multiphased study of recent clinical isolates in the United States and Australia.Diagn Microbiol Infect Dis. 2006 Jan;54(1):63-71. doi: 10.1016/j.diagmicrobio.2005.08.009. Diagn Microbiol Infect Dis. 2006. PMID: 16368476 Clinical Trial.
-
Nasal decolonization of Staphylococcus aureus with mupirocin: strengths, weaknesses and future prospects.J Antimicrob Chemother. 2009 Jul;64(1):9-15. doi: 10.1093/jac/dkp159. Epub 2009 May 18. J Antimicrob Chemother. 2009. PMID: 19451132 Free PMC article. Review.
-
Mupirocin ointment with and without chlorhexidine baths in the eradication of Staphylococcus aureus nasal carriage in nursing home residents.Am J Infect Control. 1995 Oct;23(5):306-9. doi: 10.1016/0196-6553(95)90061-6. Am J Infect Control. 1995. PMID: 8585642 Clinical Trial.
-
Clinical relevance of mupirocin resistance in Staphylococcus aureus.J Hosp Infect. 2013 Dec;85(4):249-56. doi: 10.1016/j.jhin.2013.09.006. Epub 2013 Sep 21. J Hosp Infect. 2013. PMID: 24144552 Review.
Cited by
-
Discovery of Staphylococcus aureus Adhesion Inhibitors by Automated Imaging and Their Characterization in a Mouse Model of Persistent Nasal Colonization.Microorganisms. 2021 Mar 18;9(3):631. doi: 10.3390/microorganisms9030631. Microorganisms. 2021. PMID: 33803564 Free PMC article.
-
A New Promising Anti-Infective Agent Inhibits Biofilm Growth by Targeting Simultaneously a Conserved RNA Function That Controls Multiple Genes.Antibiotics (Basel). 2021 Jan 4;10(1):41. doi: 10.3390/antibiotics10010041. Antibiotics (Basel). 2021. PMID: 33406640 Free PMC article.
-
Zinc Pyrithione Improves the Antibacterial Activity of Silver Sulfadiazine Ointment.mSphere. 2016 Sep 14;1(5):e00194-16. doi: 10.1128/mSphere.00194-16. eCollection 2016 Sep-Oct. mSphere. 2016. PMID: 27642637 Free PMC article.
-
Harnessing the Dual Antimicrobial Mechanism of Action with Fe(8-Hydroxyquinoline)3 to Develop a Topical Ointment for Mupirocin-Resistant MRSA Infections.Antibiotics (Basel). 2023 May 10;12(5):886. doi: 10.3390/antibiotics12050886. Antibiotics (Basel). 2023. PMID: 37237789 Free PMC article.
-
Protium spruceanum Extract Enhances Mupirocin Activity When Combined with Nanoemulsion-Based Hydrogel: A Multi-Target Strategy for Treating Skin and Soft Tissue Infections.Pharmaceutics. 2024 May 23;16(6):700. doi: 10.3390/pharmaceutics16060700. Pharmaceutics. 2024. PMID: 38931824 Free PMC article.
References
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi:10.1001/jama.298.15.1763. - DOI - PubMed
-
- Projan SJ, Shlaes DM. 2004. Antibacterial drug discovery: is it all downhill from here? Clin Microbiol Infect 10(Suppl 4):S18–S22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

