A Conserved Inhibitory Mechanism of a Lycorine Derivative against Enterovirus and Hepatitis C Virus
- PMID: 26596952
- PMCID: PMC4750679
- DOI: 10.1128/AAC.02274-15
A Conserved Inhibitory Mechanism of a Lycorine Derivative against Enterovirus and Hepatitis C Virus
Abstract
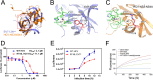
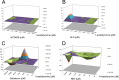
Enterovirus 71 (EV71) (Picornaviridae family) and hepatitis C virus (HCV) (Flaviviridae family) are the causative agents of human hand, foot, and mouth disease (HFMD) and hepatitis C, resulting in a severe pandemic involving millions of infections in the Asia-Pacific region and worldwide. The great impact of EV71 and HCV on public health highlights the need to further our understanding of the biology of these two viruses and develop effective therapeutic antivirals. Here, we evaluated a total of 32 lycorine derivatives and demonstrated that 1-acetyllycorine suppressed the proliferation of multiple strains of EV71 in various cells. The results of the drug resistance analysis revealed that 1-acetyllycorine targeted a phenylalanine (F76) in EV71 2A protease (2A(pro)) to stabilize the conformation of a unique zinc finger. Most interestingly, the zinc binding site in EV71 2A(pro) is the exclusive homolog of HCV NS3 among all viruses. Further analysis revealed that 1-acetyllycorine also inhibits HCV with high efficacy, and the mutation on R118 in HCV NS3, which corresponds to F76 in EV71 2A(pro), confers the resistance of HCV to 1-acetyllycorine. These results revealed a conserved mechanism of 1-acetyllycorine against EV71 and HCV through targeting viral proteases. We also documented the significant synergistic anti-EV71 and anti-HCV effects of 1-acetyllycorine with reported inhibitors, supporting potential combination therapy for the treatment of EV71 and HCV infections.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
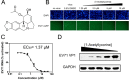
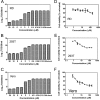
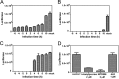
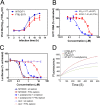
Similar articles
-
Lycorine-derived phenanthridine downregulators of host Hsc70 as potential hepatitis C virus inhibitors.Future Med Chem. 2015;7(5):561-70. doi: 10.4155/fmc.15.14. Future Med Chem. 2015. PMID: 25921398
-
Design, Synthesis and Structure-Activity Relationship Optimization of Lycorine Derivatives for HCV Inhibition.Sci Rep. 2015 Oct 7;5:14972. doi: 10.1038/srep14972. Sci Rep. 2015. PMID: 26443922 Free PMC article.
-
Inhibition of enterovirus 71 replication and viral 3C protease by quercetin.Virol J. 2018 Jul 31;15(1):116. doi: 10.1186/s12985-018-1023-6. Virol J. 2018. PMID: 30064445 Free PMC article.
-
Hepatitis C virus resistance to protease inhibitors.J Hepatol. 2011 Jul;55(1):192-206. doi: 10.1016/j.jhep.2011.01.011. Epub 2011 Feb 1. J Hepatol. 2011. PMID: 21284949 Review.
-
Future treatment of chronic hepatitis C with direct acting antivirals: is resistance important?Liver Int. 2012 Feb;32 Suppl 1:79-87. doi: 10.1111/j.1478-3231.2011.02716.x. Liver Int. 2012. PMID: 22212577 Review.
Cited by
-
An update on enterovirus 71 infection and interferon type I response.Rev Med Virol. 2019 Jan;29(1):e2016. doi: 10.1002/rmv.2016. Epub 2018 Oct 30. Rev Med Virol. 2019. PMID: 30378208 Free PMC article. Review.
-
Lycorine Induces Apoptosis of A549 Cells via AMPK-Mammalian Target of Rapamycin (mTOR)-S6K Signaling Pathway.Med Sci Monit. 2017 Apr 28;23:2035-2041. doi: 10.12659/msm.900742. Med Sci Monit. 2017. PMID: 28450693 Free PMC article.
-
Lycorine Derivative LY-55 Inhibits EV71 and CVA16 Replication Through Downregulating Autophagy.Front Cell Infect Microbiol. 2019 Aug 7;9:277. doi: 10.3389/fcimb.2019.00277. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31448243 Free PMC article.
-
Advances in the Development of Small Molecule Antivirals against Equine Encephalitic Viruses.Viruses. 2023 Feb 1;15(2):413. doi: 10.3390/v15020413. Viruses. 2023. PMID: 36851628 Free PMC article. Review.
-
Plasmids Expressing shRNAs Specific to the Nucleocapsid Gene Inhibit the Replication of Porcine Deltacoronavirus In Vivo.Animals (Basel). 2021 Apr 23;11(5):1216. doi: 10.3390/ani11051216. Animals (Basel). 2021. PMID: 33922444 Free PMC article.
References
-
- Yang Y, Wang H, Gong E, Du J, Zhao X, McNutt MA, Wang S, Zhong Y, Gao Z, Zheng J. 2009. Neuropathology in 2 cases of fatal enterovirus type 71 infection from a recent epidemic in the People's Republic of China: a histopathologic, immunohistochemical, and reverse transcription polymerase chain reaction study. Hum Pathol 40:1288–1295. doi:10.1016/j.humpath.2009.01.015. - DOI - PubMed
-
- Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, Mao N, Xu S, Zhu S, Cui A, Zhang Y, Yan D, Li Q, Dong X, Zhang J, Zhao Y, Wan J, Feng Z, Sun J, Wang S, Li D, Xu W. 2010. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J 7:94. doi:10.1186/1743-422X-7-94. - DOI - PMC - PubMed
-
- Chen C, Wang Y, Shan C, Sun Y, Xu P, Zhou H, Yang C, Shi PY, Rao Z, Zhang B, Lou Z. 2013. Crystal structure of enterovirus 71 RNA-dependent RNA polymerase complexed with its protein primer VPg: implication for a trans mechanism of VPg uridylylation. J Virol 87:5755–5768. doi:10.1128/JVI.02733-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

