An Autogenously Regulated Expression System for Gene Therapeutic Ocular Applications
- PMID: 26597678
- PMCID: PMC4656984
- DOI: 10.1038/srep17105
An Autogenously Regulated Expression System for Gene Therapeutic Ocular Applications
Erratum in
-
Author Correction: An Autogenously Regulated Expression System for Gene Therapeutic Ocular Applications.Sci Rep. 2018 Nov 23;8(1):17286. doi: 10.1038/s41598-018-35960-w. Sci Rep. 2018. PMID: 30470797 Free PMC article.
Abstract
The future of treating inherited and acquired genetic diseases will be defined by our ability to introduce transgenes into cells and restore normal physiology. Here we describe an autogenous transgene regulatory system (ARES), based on the bacterial lac repressor, and demonstrate its utility for controlling the expression of a transgene in bacteria, eukaryotic cells, and in the retina of mice. This ARES system is inducible by the small non-pharmacologic molecule, Isopropyl β-D-1-thiogalactopyranoside (IPTG) that has no off-target effects in mammals. Following subretinal injection of an adeno-associated virus (AAV) vector encoding ARES, luciferase expression can be reversibly controlled in the murine retina by oral delivery of IPTG over three induction-repression cycles. The ability to induce transgene expression repeatedly via administration of an oral inducer in vivo, suggests that this type of regulatory system holds great promise for applications in human gene therapy.
Conflict of interest statement
J. Bennett, MA. Sochor and M. Lewis are co-inventors on a US provisional patent UPN#Y6194 describing the autogenously regulated expression system. JB and JL Bennicelli (JLB) are co-inventors of US patent # X5873USA_05162011, “Proviral Plasmids for Production of Recombinant Adeno-Associated Virus,” which describes the proviral backbone plasmid used in this study. JB serves on scientific advisory boards for Avalanche Technologies, Spark Therapeutics, and Sanofi-Aventis, is a consultant for Novartis, and is a founder of GenSight Biologics. The other authors declare that they have no competing interests.
Figures
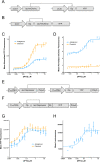

 to illustrate dynamic regulation. A fold change >1 indicates induction of luciferase expression while fold change <1 indicates repression of luciferase expression. *p < 0.05, **p < 0.01, n = 8. (F) Histological sections of injected retinas from two representative animals stained with hematoxylin and eosin. (RPE, retinal pigmented epithelium, ONL, outer nuclear layer, INL, inner nuclear layer, GC, ganglion cell layer).
to illustrate dynamic regulation. A fold change >1 indicates induction of luciferase expression while fold change <1 indicates repression of luciferase expression. *p < 0.05, **p < 0.01, n = 8. (F) Histological sections of injected retinas from two representative animals stained with hematoxylin and eosin. (RPE, retinal pigmented epithelium, ONL, outer nuclear layer, INL, inner nuclear layer, GC, ganglion cell layer).References
-
- Toniatti C., Bujard H., Cortese R. & Ciliberto G. Gene therapy progress and prospects: transcription regulatory systems. Gene Ther. 11, 649–657 (2004). - PubMed
-
- Ginn S. L., Alexander I. E., Edelstein M. L., Abedi M. R. & Wixon J. Gene therapy clinical trials worldwide to 2012 - an update. J. Gene Med. 15, 65–77 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

