Gekko japonicus genome reveals evolution of adhesive toe pads and tail regeneration
- PMID: 26598231
- PMCID: PMC4673495
- DOI: 10.1038/ncomms10033
Gekko japonicus genome reveals evolution of adhesive toe pads and tail regeneration
Abstract
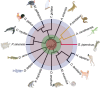
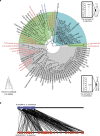
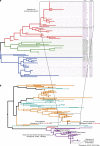
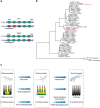
Reptiles are the most morphologically and physiologically diverse tetrapods, and have undergone 300 million years of adaptive evolution. Within the reptilian tetrapods, geckos possess several interesting features, including the ability to regenerate autotomized tails and to climb on smooth surfaces. Here we sequence the genome of Gekko japonicus (Schlegel's Japanese Gecko) and investigate genetic elements related to its physiology. We obtain a draft G. japonicus genome sequence of 2.55 Gb and annotated 22,487 genes. Comparative genomic analysis reveals specific gene family expansions or reductions that are associated with the formation of adhesive setae, nocturnal vision and tail regeneration, as well as the diversification of olfactory sensation. The obtained genomic data provide robust genetic evidence of adaptive evolution in reptiles.
Figures
References
-
- Peter U. The original descriptions of reptiles. Zootaxa 2334, 59–68 (2010) .
-
- Torstrom S. M., Pangle K. L. & Swanson B. J. Shedding subspecies: the influence of genetics on reptile subspecies taxonomy. Mol. Phylogenet. Evol. 76, 134–143 (2014) . - PubMed
-
- Vidal N. & Hedges S. B. The phylogeny of squamate reptiles (lizards, snakes, and amphisbaenians) inferred from nine nuclear protein-coding genes. C. R. Biol. 328, 1000–1008 (2005) . - PubMed
-
- Wever E. G. The lizard ear: Gekkonidae. J. Morphol. 143, 121–165 (1974) . - PubMed
Publication types
MeSH terms
Associated data
- Actions
- SRA/SRA304902
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

