The Diagnosis of Polycystic Ovary Syndrome in Adolescents
- PMID: 26598450
- PMCID: PMC9923583
- DOI: 10.1542/peds.2015-1430
The Diagnosis of Polycystic Ovary Syndrome in Adolescents
Abstract
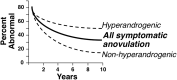
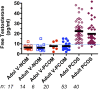
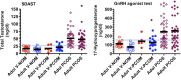
Consensus has recently been reached by international pediatric subspecialty societies that otherwise unexplained persistent hyperandrogenic anovulation using age- and stage-appropriate standards are appropriate diagnostic criteria for polycystic ovary syndrome (PCOS) in adolescents. The purpose of this review is to summarize these recommendations and discuss their basis and implications. Anovulation is indicated by abnormal uterine bleeding, which exists when menstrual cycle length is outside the normal range or bleeding is excessive: cycles outside 19 to 90 days are always abnormal, and most are 21 to 45 days even during the first postmenarcheal year. Continued menstrual abnormality in a hyperandrogenic adolescent for 1 year prognosticates at least 50% risk of persistence. Hyperandrogenism is best indicated by persistent elevation of serum testosterone above adult norms as determined in a reliable reference laboratory. Because hyperandrogenemia documentation can be problematic, moderate-severe hirsutism constitutes clinical evidence of hyperandrogenism. Moderate-severe inflammatory acne vulgaris unresponsive to topical treatment is an indication to test for hyperandrogenemia. Treatment of PCOS is symptom-directed. Cyclic estrogen-progestin oral contraceptives are ordinarily the preferred first-line medical treatment because they reliably improve both the menstrual abnormality and hyperandrogenism. First-line treatment of the comorbidities of obesity and insulin resistance is lifestyle modification with calorie restriction and increased exercise. Metformin in conjunction with behavior modification is indicated for glucose intolerance. Although persistence of hyperandrogenic anovulation for ≥2 years ensures the distinction of PCOS from physiologic anovulation, early workup is advisable to make a provisional diagnosis so that combined oral contraceptive treatment, which will mask diagnosis by suppressing hyperandrogenemia, is not unnecessarily delayed.
Copyright © 2015 by the American Academy of Pediatrics.
Conflict of interest statement
Figures



References
-
- Hart R , Doherty DA . The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab. 2015;100(3):911–919 - PubMed
-
- Rosenfield RL, Cooke DW, Radovick S. Puberty and its disorders in the female. In: Sperling M, ed. Pediatric Endocrinology. 4th ed. Philadelphia, PA: Elsevier; 2014:569–663
-
- Witchel SF, Oberfield S, Rosenfield RL, et al. The diagnosis of polycystic ovary syndrome during adolescence Horm Res Paediatr. 2015;83(6):376–389 - PubMed
-
- Vink JM , Sadrzadeh S , Lambalk CB , Boomsma DI . Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91(6):2100–2104 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

