Treatment Algorithms Based on Tumor Molecular Profiling: The Essence of Precision Medicine Trials
- PMID: 26598514
- PMCID: PMC4830395
- DOI: 10.1093/jnci/djv362
Treatment Algorithms Based on Tumor Molecular Profiling: The Essence of Precision Medicine Trials
Abstract
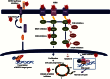
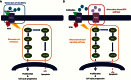
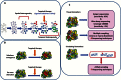
With the advent of high-throughput molecular technologies, several precision medicine (PM) studies are currently ongoing that include molecular screening programs and PM clinical trials. Molecular profiling programs establish the molecular profile of patients' tumors with the aim to guide therapy based on identified molecular alterations. The aim of prospective PM clinical trials is to assess the clinical utility of tumor molecular profiling and to determine whether treatment selection based on molecular alterations produces superior outcomes compared with unselected treatment. These trials use treatment algorithms to assign patients to specific targeted therapies based on tumor molecular alterations. These algorithms should be governed by fixed rules to ensure standardization and reproducibility. Here, we summarize key molecular, biological, and technical criteria that, in our view, should be addressed when establishing treatment algorithms based on tumor molecular profiling for PM trials.
© The Author 2015. Published by Oxford University Press.
Figures
References
-
- Sleijfer S, Ballman K, Verweij J. The future of drug development? Seeking evidence of activity of novel drugs in small groups of patients. J Clin Oncol. 2013;31(18):2246–2248. - PubMed
-
- Le Tourneau C, Kamal M, Alt M, et al. The spectrum of clinical trials aiming at personalizing medicine. Chin Clin Oncol. 2014;3(2):13. - PubMed
-
- Le Tourneau C, Paoletti X, Servant N, et al. Randomised proof-of-concept phase II trial comparing targeted therapy based on tumour molecular profiling vs conventional therapy in patients with refractory cancer: results of the feasibility part of the SHIVA trial. Br J Cancer. 2014;111(1):17–24. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

