Phase II trial of dasatinib for recurrent or metastatic c-KIT expressing adenoid cystic carcinoma and for nonadenoid cystic malignant salivary tumors
- PMID: 26598548
- PMCID: PMC4722891
- DOI: 10.1093/annonc/mdv537
Phase II trial of dasatinib for recurrent or metastatic c-KIT expressing adenoid cystic carcinoma and for nonadenoid cystic malignant salivary tumors
Abstract
Background: Adenoid cystic carcinoma (ACC) is a subtype of malignant salivary gland tumors (MSGT), in which 90% of cases express cKIT. Dasatinib is a potent and selective inhibitor of five oncogenic protein tyrosine kinases (PTKs)/kinase families including cKIT. We conducted a phase II study to determine the antitumor activity of dasatinib in ACC and non-ACC MSGT.
Patients and methods: In a two-stage design, patients with progressive, recurrent/metastatic ACC (+cKIT) and non-ACC MSGT (separate cohort) were treated with dasatinib 70 mg p.o. b.i.d. Response was assessed every 8 weeks using RECIST.
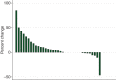
Results: Of 54 patients: 40 ACC, 14 non-ACC (1, ineligible excluded); M:F = 28 : 26, median age 56 years (range 20-82 years), ECOG performance status 0 : 1 : 2 = 24 : 28 : 2, prior radiation: 44, prior chemotherapy: 21. The most frequent adverse events (AEs) (as % of patients, worst grade 2 or higher) were: fatigue (28%), nausea (19%), headache (15%), lymphopenia (7%), dyspnea (11%), alanine aminotransferase increased (7%), anorexia (7%), vomiting (7%), alkaline phosphatase increased (6%), diarrhea (6%), neutropenia (6%), and noncardiac chest pain (6%). No grade 4 AE occurred, 15 patients experienced a grade 3 AE, primarily dyspnea (5) and fatigue (4), and cardiac toxicity (1 prolonged QTc). Among ACC patients, best response to dasatinib: 1 patient (2.5%) had partial response, 20 patients (50%) had stable disease (SD) (3-14 months), 12 patients (30%) had PD, 2 withdrew, 3 discontinued therapy due to AE, and 2 died before cycle 2. Median progression-free survival was 4.8 months. Median overall survival was 14.5 months. For 14 assessable non-ACC patients, none had objective response, triggering early stopping rule. Seven had SD (range 1-7 months), 4 PD, 2 discontinued therapy due to AE, and 1 died before cycle 2.
Conclusion: Although there was only one objective response, dasatinib is well tolerated, with tumor stabilization achieved by 50% of ACC patients. Dasatinib demonstrated no activity in non-ACC MSGT.
Keywords: adenoid cystic carcinoma; cKIT; dasatinib; malignant salivary gland cancer; phase II.
© The Author 2015. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Speight PM, Barrett AW. Salivary gland tumours. Oral Dis 2002; 8(5): 229–240. - PubMed
-
- Seifert G, Brocheriou C, Cardesa A, Eveson JW. WHO International Histological Classification of Tumours. Tentative histological classification of salivary gland tumours. Pathol Res Pract 1990; 186(5): 555–581. - PubMed
-
- Dimery IW, Legha SS, Shirinian M, Hong WK. Fluorouracil, doxorubicin, cyclophosphamide, and cisplatin combination chemotherapy in advanced or recurrent salivary gland carcinoma. J Clin Oncol 1990; 8(6): 1056–1062. - PubMed
-
- Licitra L, Marchini S, Spinazze S et al. . Cisplatin in advanced salivary gland carcinoma. A phase II study of 25 patients. Cancer 1991; 68(9): 1874–1877. - PubMed
-
- Gilbert J, Li Y, Pinto HA et al. . Phase II trial of taxol in salivary gland malignancies (E1394): a trial of the Eastern Cooperative Oncology Group. Head Neck 2006; 28(3): 197–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

