p53 Restoration in Induction and Maintenance of Senescence: Differential Effects in Premalignant and Malignant Tumor Cells
- PMID: 26598601
- PMCID: PMC4719431
- DOI: 10.1128/MCB.00747-15
p53 Restoration in Induction and Maintenance of Senescence: Differential Effects in Premalignant and Malignant Tumor Cells
Abstract
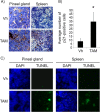
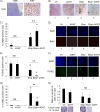
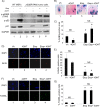
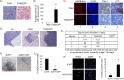
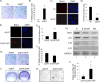
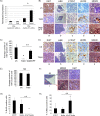
The restoration of p53 has been suggested as a therapeutic approach in tumors. However, the timing of p53 restoration in relation to its efficacy during tumor progression still is unclear. We now show that the restoration of p53 in murine premalignant proliferating pineal lesions resulted in cellular senescence, while p53 restoration in invasive pineal tumors did not. The effectiveness of p53 restoration was not dependent on p19(Arf) expression but showed an inverse correlation with Mdm2 expression. In tumor cells, p53 restoration became effective when paired with either DNA-damaging therapy or with nutlin, an inhibitor of p53-Mdm2 interaction. Interestingly, the inactivation of p53 after senescence resulted in reentry into the cell cycle and rapid tumor progression. The evaluation of a panel of human supratentorial primitive neuroectodermal tumors (sPNET) showed low activity of the p53 pathway. Together, these data suggest that the restoration of the p53 pathway has different effects in premalignant versus invasive pineal tumors, and that p53 activation needs to be continually sustained, as reversion from senescence occurs rapidly with aggressive tumor growth when p53 is lost again. Finally, p53 restoration approaches may be worth exploring in sPNET, where the p53 gene is intact but the pathway is inactive in the majority of examined tumors.
Copyright © 2016 Harajly et al.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
