Formamide reaction network in gas phase and solution via a unified theoretical approach: Toward a reconciliation of different prebiotic scenarios
- PMID: 26598679
- PMCID: PMC4679036
- DOI: 10.1073/pnas.1512486112
Formamide reaction network in gas phase and solution via a unified theoretical approach: Toward a reconciliation of different prebiotic scenarios
Abstract
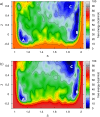
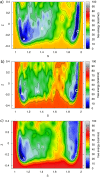
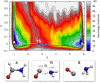
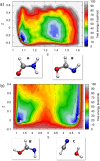
Increasing experimental and theoretical evidence points to formamide as a possible hub in the complex network of prebiotic chemical reactions leading from simple precursors like H2, H2O, N2, NH3, CO, and CO2 to key biological molecules like proteins, nucleic acids, and sugars. We present an in-depth computational study of the formation and decomposition reaction channels of formamide by means of ab initio molecular dynamics. To this aim we introduce a new theoretical method combining the metadynamics sampling scheme with a general purpose topological formulation of collective variables able to track a wide range of different reaction mechanisms. Our approach is flexible enough to discover multiple pathways and intermediates starting from minimal insight on the systems, and it allows passing in a seamless way from reactions in gas phase to reactions in liquid phase, with the solvent active role fully taken into account. We obtain crucial new insight into the interplay of the different formamide reaction channels and into environment effects on pathways and barriers. In particular, our results indicate a similar stability of formamide and hydrogen cyanide in solution as well as their relatively facile interconversion, thus reconciling experiments and theory and, possibly, two different and competing prebiotic scenarios. Moreover, although not explicitly sought, formic acid/ammonium formate is produced as an important formamide decomposition byproduct in solution.
Keywords: ab initio molecular dynamics; chemical reactions; formamide; free energy landscapes; prebiotic scenarios.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


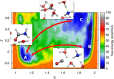
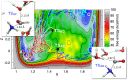
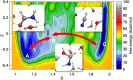
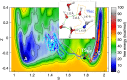
References
-
- Darwin CD. Letter to Joseph Hooker [7471] Correspondence of Charles Darwin. 1871;19:188–189.
-
- Pilling S, Baptista L, Boechat-Roberty HM, Andrade DP. Formation routes of interstellar glycine involving carboxylic acids: Possible favoritism between gas and solid phase. Astrobiology. 2011;11(9):883–893. - PubMed
-
- Kahane C, Ceccarelli C, Faure A, Caux E. Detection of formamide, the simplest but crucial amide in a solar type protostar. Astrophys J. 2013;763(2):L38.
-
- Lambert J-F. Adsorption and polymerization of amino acids on mineral surfaces: A review. Orig Life Evol Biosph. 2008;38(3):211–242. - PubMed
-
- Bar-Nun A, Bar-Nun N, Bauer SH, Sagan C. Shock synthesis of amino acids in simulated primitive environments. Science. 1970;168(3930):470–473. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

