The peptide agonist-binding site of the glucagon-like peptide-1 (GLP-1) receptor based on site-directed mutagenesis and knowledge-based modelling
- PMID: 26598711
- PMCID: PMC4718506
- DOI: 10.1042/BSR20150253
The peptide agonist-binding site of the glucagon-like peptide-1 (GLP-1) receptor based on site-directed mutagenesis and knowledge-based modelling
Abstract
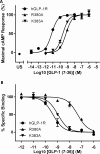
Glucagon-like peptide-1 (7-36)amide (GLP-1) plays a central role in regulating blood sugar levels and its receptor, GLP-1R, is a target for anti-diabetic agents such as the peptide agonist drugs exenatide and liraglutide. In order to understand the molecular nature of the peptide-receptor interaction, we used site-directed mutagenesis and pharmacological profiling to highlight nine sites as being important for peptide agonist binding and/or activation. Using a knowledge-based approach, we constructed a 3D model of agonist-bound GLP-1R, basing the conformation of the N-terminal region on that of the receptor-bound NMR structure of the related peptide pituitary adenylate cyclase-activating protein (PACAP21). The relative position of the extracellular to the transmembrane (TM) domain, as well as the molecular details of the agonist-binding site itself, were found to be different from the model that was published alongside the crystal structure of the TM domain of the glucagon receptor, but were nevertheless more compatible with published mutagenesis data. Furthermore, the NMR-determined structure of a high-potency cyclic conformationally-constrained 11-residue analogue of GLP-1 was also docked into the receptor-binding site. Despite having a different main chain conformation to that seen in the PACAP21 structure, four conserved residues (equivalent to His-7, Glu-9, Ser-14 and Asp-15 in GLP-1) could be structurally aligned and made similar interactions with the receptor as their equivalents in the GLP-1-docked model, suggesting the basis of a pharmacophore for GLP-1R peptide agonists. In this way, the model not only explains current mutagenesis and molecular pharmacological data but also provides a basis for further experimental design.
Keywords: GLP-1; GPCR; diabetes; hormone; molecular model; receptor.
© 2016 Authors.
Figures




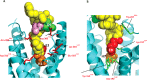
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

