Overview of current immunotherapeutic strategies for glioma
- PMID: 26598957
- PMCID: PMC4681396
- DOI: 10.2217/imt.15.75
Overview of current immunotherapeutic strategies for glioma
Abstract
In the last decade, numerous studies of immunotherapy for malignant glioma (glioblastoma multiforme) have brought new knowledge and new hope for improving the prognosis of this incurable disease. Some clinical trials have reached Phase III, following positive outcomes in Phase I and II, with respect to safety and immunological end points. Results are encouraging especially when considering the promise of sustained efficacy by inducing antitumor immunological memory. Progress in understanding the mechanisms of tumor-induced immune suppression led to the development of drugs targeting immunosuppressive checkpoints, which are used in active clinical trials for glioblastoma multiforme. Insights related to the heterogeneity of the disease bring new challenges for the management of glioma and underscore a likely cause of therapeutic failure. An emerging therapeutic strategy is represented by a combinatorial, personalized approach, including the standard of care: surgery, radiation, chemotherapy with added active immunotherapy and multiagent targeting of immunosuppressive checkpoints.
Keywords: clinical trials; dendritic cells; gene therapy; glioma; immune checkpoints blockade; immunotherapy; vaccination.
Conflict of interest statement
Financial & competing interests disclosure This work was supported by NIH/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants RO1-NS094804, R01-NS074387, R01-NS057711, R21-NS091555, University of Michigan U042841 to MG Castro; NIH/NINDS Grants R01-NS054193, R01- NS061107, R01-NS082311 and R21-NS084275 to PR Lowenstein; the Michigan Institute for Clinical and Health Research, NIH 2UL1-TR000433; University of Michigan Cancer Biology Training Grant, NIH/NCI (National Cancer Institute) T32-CA009676; University of Michigan Training in Clinical and Basic Neuroscience, NIH/NINDS T32-NS007222 and the University of Michigan Medical Scientist Training Program, NIH/NIGMS (National Institute of General Medicine Sciences) T32. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures
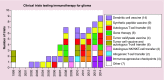

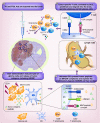

References
-
- Greene HS. Heterotransplantation of tumors. Ann. NY Acad. Sci. 1957;69(4):818–829. - PubMed
-
- Baldwin RW, Robins RA. Factors interfering with immunological rejection of tumours. Br. Med. Bull. 1976;32(2):118–123. - PubMed
-
- Burnet FM. Immunological aspects of malignant disease. Lancet. 1967;1(7501):1171–1174. - PubMed
-
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011;29:235–271. - PubMed
Publication types
MeSH terms
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- R21-NS084275/NS/NINDS NIH HHS/United States
- R01 NS054193/NS/NINDS NIH HHS/United States
- R01-NS054193/NS/NINDS NIH HHS/United States
- R01 NS082311/NS/NINDS NIH HHS/United States
- R01 NS074387/NS/NINDS NIH HHS/United States
- R01 NS061107/NS/NINDS NIH HHS/United States
- R01-NS082311/NS/NINDS NIH HHS/United States
- R21 NS084275/NS/NINDS NIH HHS/United States
- T32 CA009676/CA/NCI NIH HHS/United States
- T32 NS007222/NS/NINDS NIH HHS/United States
- R01-NS057711/NS/NINDS NIH HHS/United States
- R01 NS094804/NS/NINDS NIH HHS/United States
- 2UL1-TR000433/TR/NCATS NIH HHS/United States
- R21-NS091555/NS/NINDS NIH HHS/United States
- R37 NS094804/NS/NINDS NIH HHS/United States
- R01- NS061107/NS/NINDS NIH HHS/United States
- R01 NS057711/NS/NINDS NIH HHS/United States
- R01-NS074387/NS/NINDS NIH HHS/United States
- R21 NS091555/NS/NINDS NIH HHS/United States
- T32-CA009676/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
