A Novel MVA-Based Multiphasic Vaccine for Prevention or Treatment of Tuberculosis Induces Broad and Multifunctional Cell-Mediated Immunity in Mice and Primates
- PMID: 26599077
- PMCID: PMC4658014
- DOI: 10.1371/journal.pone.0143552
A Novel MVA-Based Multiphasic Vaccine for Prevention or Treatment of Tuberculosis Induces Broad and Multifunctional Cell-Mediated Immunity in Mice and Primates
Abstract
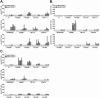
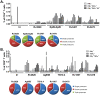
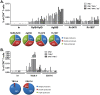
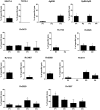
Bacille Calmette-Guérin (BCG) vaccination of new born babies can protect children against tuberculosis (TB), but fails to protect adults consistently against pulmonary TB underlying the urgent need to develop novel TB vaccines. Majority of first generation TB vaccine candidates have relied on a very limited number of antigens typically belonging to the active phase of infection. We have designed a multi-antigenic and multiphasic vaccine, based on the Modified Vaccinia Ankara virus (MVA). Up to fourteen antigens representative of the three phases of TB infection (active, latent and resuscitation) were inserted into MVA. Using three different strains of mouse (BALB/c, C57BL/6 and C3H/HeN), we show that a single vaccination results in induction of both CD4 and CD8 T cells, displaying capacity to produce multiple cytokines together with cytolytic activity targeting a large array of epitopes. As expected, dominance of responses was linked to the mouse haplotype although for a given haplotype, responses specific of at least one antigen per phase could always be detected. Vaccination of non-human primates with the 14 antigens MVA-TB candidate resulted in broad and potent cellular-based immunogenicity. The remarkable plasticity of MVA opens the road to development of a novel class of highly complex recombinant TB vaccines to be evaluated in both prophylactic and therapeutic settings.
Conflict of interest statement
Figures

References
-
- WHO. Global tuberculosis report. WHO, Geneva, Switzerland. 2014.
-
- Marx FM, Dunbar R, Enarson DA, Williams BG, Warren RM, van der Spuy GD, et al. The temporal dynamics of relapse and reinfection tuberculosis after successful treatment: a retrospective cohort study. Clin Infect Dis. 2014;58(12):1676–83. Epub 2014 Mar 18. - PubMed
-
- Schiroli C, Carugati M, Zanini F, Bandera A, Di Nardo Stuppino S, Monge E, et al. Exogenous reinfection of tuberculosis in a low-burden area. Infection. 2015;10:10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

