A Risk Assessment of the Jaffe vs Enzymatic Method for Creatinine Measurement in an Outpatient Population
- PMID: 26599086
- PMCID: PMC4657986
- DOI: 10.1371/journal.pone.0143205
A Risk Assessment of the Jaffe vs Enzymatic Method for Creatinine Measurement in an Outpatient Population
Abstract
Background: The Jaffe and enzymatic methods are the two most common methods for measuring serum creatinine. The Jaffe method is less expensive than the enzymatic method but is also more susceptible to interferences. Interferences can lead to misdiagnosis but interferences may vary by patient population. The overall risk associated with the Jaffe method depends on the probability of misclassification and the consequences of misclassification. This study assessed the risk associated with the Jaffe method in an outpatient population. We analyzed the discordance rate in the estimated glomerular filtration rate based on serum creatinine measurements obtained by the Jaffe and enzymatic method.
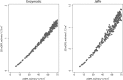
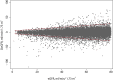
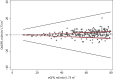
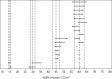
Methods: Method comparison and risk analysis. Five hundred twenty-nine eGFRs obtained by the Jaffe and enzymatic method were compared at four clinical decision limits. We determined the probability of discordance and the consequence of misclassification at each decision limit to evaluate the overall risk.
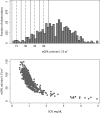
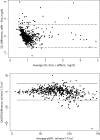
Results: We obtained 529 paired observations. Of these, 29 (5.5%) were discordant with respect to one of the decision limits (i.e. 15, 30, 45 or 60 ml/min/1.73m2). The magnitude of the differences (Jaffe result minus enzymatic result) were significant relative to analytical variation in 21 of the 29 (72%) of the discordant results. The magnitude of the differences were not significant relative to biological variation. The risk associated with misclassification was greatest at the 60 ml/min/1.73m2 decision limit because the probability of misclassification and the potential for adverse outcomes were greatest at that decision limit.
Conclusion: The Jaffe method is subject to bias due to interfering substances (loss of analytical specificity). The risk of misclassification is greatest at the 60 ml/min/1.73m2 decision limit; however, the risk of misclassification due to bias is much less than the risk of misclassification due to biological variation. The Jaffe method may pose low risk in selected populations if eGFR results near the 60 ml/min/1.73m2 decision limit are interpreted with caution.
Conflict of interest statement
Figures
References
-
- Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52(1):5–18. Epub 2005/12/08. 10.1373/clinchem.2005.0525144 . - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

