Functional Characterization of Germline Mutations in PDGFB and PDGFRB in Primary Familial Brain Calcification
- PMID: 26599395
- PMCID: PMC4658112
- DOI: 10.1371/journal.pone.0143407
Functional Characterization of Germline Mutations in PDGFB and PDGFRB in Primary Familial Brain Calcification
Abstract
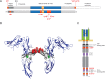
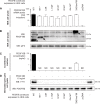
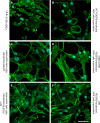
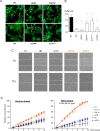
Primary Familial Brain Calcification (PFBC), a neurodegenerative disease characterized by progressive pericapillary calcifications, has recently been linked to heterozygous mutations in PDGFB and PDGFRB genes. Here, we functionally analyzed several of these mutations in vitro. All six analyzed PDGFB mutations led to complete loss of PDGF-B function either through abolished protein synthesis or through defective binding and/or stimulation of PDGF-Rβ. The three analyzed PDGFRB mutations had more diverse consequences. Whereas PDGF-Rβ autophosphorylation was almost totally abolished in the PDGFRB L658P mutation, the two sporadic PDGFRB mutations R987W and E1071V caused reductions in protein levels and specific changes in the intensity and kinetics of PLCγ activation, respectively. Since at least some of the PDGFB mutations were predicted to act through haploinsufficiency, we explored the consequences of reduced Pdgfb or Pdgfrb transcript and protein levels in mice. Heterozygous Pdgfb or Pdgfrb knockouts, as well as double Pdgfb+/-;Pdgfrb+/- mice did not develop brain calcification, nor did Pdgfrbredeye/redeye mice, which show a 90% reduction of PDGFRβ protein levels. In contrast, Pdgfbret/ret mice, which have altered tissue distribution of PDGF-B protein due to loss of a proteoglycan binding motif, developed brain calcifications. We also determined pericyte coverage in calcification-prone and non-calcification-prone brain regions in Pdgfbret/ret mice. Surprisingly and contrary to our hypothesis, we found that the calcification-prone brain regions in Pdgfbret/ret mice model had a higher pericyte coverage and a more intact blood-brain barrier (BBB) compared to non-calcification-prone brain regions. While our findings provide clear evidence that loss-of-function mutations in PDGFB or PDGFRB cause PFBC, they also demonstrate species differences in the threshold levels of PDGF-B/PDGF-Rβ signaling that protect against small-vessel calcification in the brain. They further implicate region-specific susceptibility factor(s) in PFBC pathogenesis that are distinct from pericyte and BBB deficiency.
Conflict of interest statement
Figures



References
-
- Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126: 3047–3055. - PubMed
-
- Levéen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes & Development. 1994;8: 1875–1887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

