Asenapine versus placebo for schizophrenia
- PMID: 26599405
- PMCID: PMC6464872
- DOI: 10.1002/14651858.CD011458.pub2
Asenapine versus placebo for schizophrenia
Abstract
Background: Schizophrenia is a highly prevalent and chronic disorder that comprises a wide range of symptomatology. Asenapine is a recently developed atypical antipsychotic that is approved by the US Food and Drug Administration (FDA) for the treatment of schizophrenia.
Objectives: To determine the clinical effects of asenapine for adults with schizophrenia or other schizophrenia-like disorders by comparing it with placebo.
Search methods: We searched the Cochrane Schizophrenia Group's Trials Register (July 04, 2014) which is based on regular searches of MEDLINE, EMBASE, CINAHL, BIOSIS, AMED, PubMed, PsycINFO, and registries of clinical trials. There are no language, date, document type, or publication status limitation for inclusion of records into the register. We inspected references of all included studies for further relevant studies.
Selection criteria: Our review includes randomised controlled trials (RCTs) comparing asenapine with placebo in adults (however defined) with schizophrenia or related disorders, including schizophreniform disorder, schizoaffective disorder and delusional disorder, again, by any means of diagnosis.
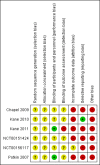
Data collection and analysis: We inspected citations from the searches and identified relevant abstracts, and extracted data from all included studies. For binary data we calculated risk ratio (RR) with 95% confidence intervals (CI), and for continuous data we calculated mean differences (MD). We used the GRADE approach to produce a 'Summary of findings' table which included our outcomes of interest, where possible. We used a fixed-effect model for our analyses.
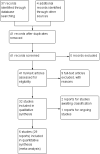
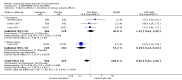
Main results: We obtained and scrutinised 41 potentially relevant records, and from these we could include only six trials (n = 1835). Five of the six trials had high risk of attrition bias and all trials were sponsored by pharmaceutical companies. Results showed a clinically important change in global state (1 RCT, n = 336, RR 0.81, 95% CI 0.68 to 0.97, low-quality evidence) and mental state (1 RCT, n = 336, RR 0.72, 95% CI 0.59 to 0.86, very low-quality evidence) at short-term amongst people receiving asenapine. People receiving asenapine demonstrated significant reductions in negative symptoms (1 RCT, n = 336, MD -1.10, 95% CI -2.29 to 0.09, very low-quality evidence) at short-term. Individuals receiving asenapine demonstrated significantly fewer incidents of serious adverse effects (1 RCT, n = 386, RR 0.29, 95% CI 0.14 to 0.63, very low-quality evidence) at medium-term. There was no clear difference in people discontinuing the study for any reason between asenapine and placebo at short-term (5 RCTs, n = 1046, RR 0.91, 95% CI 0.80 to 1.04, very low-quality evidence). No trial reported data for extrapyramidal symptoms or costs.
Authors' conclusions: There is some, albeit preliminary, evidence that asenapine provides an improvement in positive, negative, and depressive symptoms, whilst minimising the risk of adverse effects. However due to the low-quality and limited quantity of evidence, it remains difficult to recommend the use of asenapine for people with schizophrenia. We identify a need for large-scale, longer-term, better-designed and conducted randomised controlled trials investigating the clinical effects and safety of asenapine.
Conflict of interest statement
Alistair Hay – none known.
Amy Byers – none known.
Marco Sereno – none known.
Manpreet Basra – none known.
Snigdha Dutta – none known.
Figures





















Update of
References
References to studies included in this review
Chapel 2009 {published data only}
-
- Chapel S, Hutmacher MM, Haig G, Bockbrader H, Greef R, Preskorn SH, et al. Exposure‐response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. Journal of Clinical Pharmacology 2009;49(11):1297‐308. - PubMed
-
- Preskorn AS, Chapel S, Panagides J. Effect of asenapine versus quetiapine and placebo on QTc interval in patients with schizophrenia. European Neuropsychopharmacology 2007;17(Suppl 4):S453.
-
- Preskorn SH, Chapel S, Panagides J. Effects of asenapine versus placebo on QTc interval in patients with schizophrenia. American Psychiatric Association, 161st Annual Meeting, May 3‐8, 2008, Washington, DC. 2008.
Kane 2010 {published data only}
-
- Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second‐generation antipsychotic. The International Journal of Clinical Practice 2009;63:1762‐84. - PubMed
-
- Kane J, Zhao J, Cohen M, Panagides J. Efficacy and safety of asenapine in patients with acute exacerbation of schizophrenia. Schizophrenia Research 2008;98:14. - PubMed
-
- Kane JM, Cohen M, Zhao J, Alphs L, Panagides J. Efficacy and safety of asenapine in a placebo‐ and haloperidol‐controlled trial in patients with acute exacerbation of schizophrenia. Journal of Clinical Psychopharmacology 2010;30(2):106‐15. - PubMed
-
- Kane JM, Zhao J, Cohen M, Panagides J. Efficacy and safety of asenapine in patients with acute schizophrenia. American Psychiatric Association, 161st Annual Meeting, May 3‐8, 2008, Washington, DC. 2008.
-
- NCT00156104. A multicenter, randomized, double‐blind, fixed‐dose, 6‐week trial of the efficacy and safety of asenapine compared with placebo using haloperidol positive control in subjects with an acute exacerbation of schizophrenia. http://clinicaltrials.gov/show/NCT00156104 2008.
Kane 2011 {published data only}
-
- Kane JM, Mackle M, Snow‐Adami L, Zhao J, Szegedi A, Panagides J. A randomized placebo‐controlled trial of asenapine for the prevention of relapse of schizophrenia after long‐term treatment. Journal of Clinical Psychiatry 2011;72(3):349‐55. - PubMed
-
- Kane JM, Mackle M, Snow‐Adami L, Zhao J, Szegedi A, Panagides J. Double‐blind, placebo‐controlled trial of asenapine in prevention of relapse after long‐term treatment of schizophrenia. International Journal of Neuropsychopharmacology 2010;13:223.
-
- Mackle M, Snow‐Adami L, Zhao J, Szegedi A, Panagides J. Double‐blind, placebo‐controlled trial of asenapine in prevention of relapse after long‐term treatment of schizophrenia. European Neuropsychopharmacology. 2009; Vol. Conference Start: 20090912 Conference End: 20090916. Conference Publication:, issue Suppl 3:pp S543.
-
- NCT00150176. A randomized, placebo‐controlled, double‐blind trial of asenapine in the prevention of relapse after long‐term treatment of schizophrenia. http://clinicaltrials.gov/show/NCT00150176 2005.
NCT00151424 {published data only}
-
- Citrome L. Asenapine for schizophrenia and bipolar disorder: A review of the efficacy and safety profile for this newly approved sublingually absorbed second‐generation antipsychotic. The International Journal of Clinical Practice 2009;63:1762‐84. - PubMed
-
- NCT00151424. A multicenter, randomized, double‐blind, flexible‐dose, 6‐week trial of the efficacy and safety of asenapine compared with placebo using olanzapine positive control in subjects with an acute exacerbation of schizophrenia. http://clinicaltrials.gov/show/NCT00151424 2005.
-
- Potkin SG, Kane JM, Emsley RA, Naber D, Panagides J. Asenapine in schizophrenia: an overview of clinical trials in the Olympia program. Society of Biological Psychiatry, 63rd Annual Scientific Convention and Meeting, May 1‐3, 2008, Washington, DC. 2008.
-
- Szegedi A, Verweij P, Duijnhoven W, Mackle M, Cazorla P, Fennema H. Meta‐analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychotics. Journal of Clinical Psychiatry 2012;73:1533‐40. - PubMed
NCT00156117 {published data only}
-
- Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second‐generation antipsychotic. The International Journal of Clinical Practice 2009;63:1762‐84. - PubMed
-
- Leucht S, Zhao J. Early improvement as a predictor of treatment response and remission in patients with acute schizophrenia: effects of asenapine. European Neuropsychopharmacology 2013; Vol. 23:S470.
-
- Leucht S, Zhao J. Early improvement as a predictor of treatment response and remission in patients with schizophrenia: a pooled, post‐hoc analysis from the asenapine development program. Journal of Psychopharmacology 2014;28:387‐94. - PubMed
-
- NCT00156117. A multicenter, randomized, double‐blind, fixed‐dose, 6‐week trial of the efficacy and safety of asenapine compared with placebo using olanzapine positive control in subjects with an acute exacerbation of schizophrenia. http://clinicaltrials.gov/show/NCT00156117 2005.
-
- Potkin SG, Kane JM, Emsley RA, Naber D, Panagides J. Asenapine in schizophrenia: an overview of clinical trials in the Olympia program. Society of Biological Psychiatry, 63rd Annual Scientific Convention and Meeting, May 1‐3, 2008, Washington, DC. 2008.
Potkin 2007 {published data only}
-
- Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second‐generation antipsychotic. The International Journal of Clinical Practice 2009;63:1762‐84. - PubMed
-
- Fleming K, Potkin SG, Binneman B, Keller D, Alphs L, Panagides J. Effects of asenapine on cognitive function in acute schizophrenia: a placebo‐ and risperidone‐controlled trial. European Neuropsychopharmacology 2007;17(Suppl 4):S466.
-
- Potkin S, Fleming K, Binneman B, Keller DS, Alphs L, Panagides J. Asenapine improves cognitive function in acute schizophrenia: a placebo‐ and risperidone‐ controlled trial. Proceedings of the 160th Annual Meeting of the American Psychiatric Association; 2007 May 19‐24; San Diego, CA. 2007.
-
- Potkin S, Fleming K, Binneman B, Keller S. Asenapine cognitive function effects in acute schizophrenia: a placebo‐and risperidone‐controlled trial. Schizophrenia Bulletin 2007;33(2):454.
-
- Potkin SG, Cohen M, Baker RA, Jina AS, Nettler S, Alphs L, et al. Asenapine, a novel psychotherapeutic agent with efficacy in positive and negative symptoms during acute episodes of schizophrenia: a randomized, placebo‐ and risperidone‐controlled trial. Neuropsychopharmacology 2005;30(Suppl 1):S112‐3.
References to studies excluded from this review
Castle 2013 {published data only}
-
- Castle D, Jensen JKS. Management of depressive symptoms in schizophrenia: a pooled, post hoc analysis from the asenapine development program. (Manuscript submitted for peer‐review, journal not currently known).
-
- Castle D, Jensen JKS. Management of depressive symptoms in schizophrenia: a pooled, post hoc analysis from the asenapine development program. European Neuropsychopharmacology 2013;23:S502‐3.
Cazorla 2008 {published data only}
-
- Cazorla P, Panagides J, Alphs L, Kouassi A, Buchanan R. Asenapine versus olanzapine in patients with predominant, persistent negative symptoms of schizophrenia. American Psychiatric Association, 161st Annual Meeting, May 3‐8, 2008, Washington, DC. 2008.
-
- Cazorla P, Panagides J, Alphs L, Kouassi A, Buchanan R, Szegedi A. Asenapine versus olanzapine in patients with predominant, persistent negative symptoms of schizophrenia. International Journal of Neuropsychopharmacology 2008;11(Suppl 1):138‐9.
NCT00156065 {published data only}
-
- Meltzer H, Cohen M, Snow‐Adami L, Mackle M, Zhao J, Szegedi A, et al. Long‐term safety and maintenance of effect of asenapine in patients with acute exacerbation of schizophrenia. European Neuropsychopharmacology 2009;19:S536‐S537.
-
- NCT00156065. A multicenter, double‐blind, flexible dose, long‐term extension trial of the safety and maintenance of effect of asenapine using a haloperidol positive control in subjects who complete protocol 041023. http://clinicaltrials.gov/show/NCT00156065 2005.
NCT01142596 {published data only}
-
- NCT01142596. Long‐term extension trial of asenapine in subjects with schizophrenia (study p06125). http://clinicaltrials.gov/show/NCT01142596 2010.
NCT01190254 {published data only}
-
- NCT01190254. Fixed dose efficacy and safety study of asenapine for the treatment of schizophrenia in adolescents (study p05896). http://clinicaltrials.gov/show/NCT01190254 2010.
The National Horizon Scanning Centre 2010 {published data only}
-
- The National Horizon Scanning Centre. Asenapine (Saphris) for schizophrenia. The National Horizon Scanning Centre, Department of Public Health and Epidemiology, University of Birmingham. University of Birmingham, 2010.
References to studies awaiting assessment
NCT00156091 {published data only}
-
- NCT00156091. A multicenter, double‐blind, flexible‐dose, long‐term extension trial of the safety and maintenance of effect of asenapine using olanzapine positive control in subjects who complete protocols 041021/041022. http://clinicaltrials.gov/show/NCT00156091 2005.
NCT01098110 {published data only}
-
- Nct. 6‐week trial of the efficacy and safety of asenapine compared to placebo in subjects with an acute exacerbation of schizophrenia (Study P06124). http://clinicaltrials.gov/show/NCT01098110 2010.
References to ongoing studies
NCT01617187 {published data only}
-
- NCT01617187. A study of the efficacy and safety of asenapine in participants with an acute exacerbation of schizophrenia (p05688 am2). http://clinicaltrials.gov/show/NCT01617187 2012.
Additional references
Abi‐Dargham 2004
-
- Abi‐Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. International Journal of Neuropsychopharmacology 2004;7:S1‐5. - PubMed
Adams 2013
Addington 1993
-
- Addington D, Addington J, Maticka‐Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. The British Journal of Psychiatry 1993;163:39‐44. - PubMed
Altman 1996
Andreasen 1999
-
- Andreasen NC. A unitary model of schizophrenia. Bleuler's "fragmented phrene" as schizencephaly. Archives of General Psychiatry 1999;56:781‐7. - PubMed
Ayuso‐Mateos 2006
-
- Ayuso‐Mateos JL. Global burden of schizophrenia in the year 2000. http://www.who.int/healthinfo/statistics/bod_schizophrenia.pdf 2006.
Barnes 1989
-
- Barnes TR. A rating scale for drug‐induced akathisia. The British Journal of Psychiatry 1989;154:672‐6. - PubMed
Bishara 2009
Bland 1997
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacité thérapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacité]. Thérapie 1999;54(4):405‐11. [PUBMED: 10667106] - PubMed
Campbell 1999
Citrome 2009
-
- Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second‐generation antipsychotic. The International Journal of Clinical Practice 2009;63:1762‐84. - PubMed
Crow 1980
Davis 2003
-
- Davis JM, Chen N, Glick ID. A meta‐analysis of the efficacy of second‐generation antipsychotics. Archives of General Psychiatry 2003;60:553‐64. - PubMed
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Divine 1992
-
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Duggan 2005
Egger 1997
Elbourne 2002
-
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
EMA 2014
-
- European Medicines Agency. Sycrest: EPAR ‐ Product information. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Info... 2014.
Emsley 2013
-
- Emsley R, Fleischhacker WW. Is the ongoing use of placebo in relapse‐prevention clinical trials in schizophrenia justified?. Schizophrenia Research 2013;150:427‐33. - PubMed
FDA 2013
-
- Food, Drug Administration. Saphris (asenapine) prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022117s012lbl.pdf 2013.
Fioravanti 2005
-
- Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta‐analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychology Review 2005;15:73‐95. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. - PubMed
Gulliford 1999
-
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy W. ECDEU Asessment Manual for Psychopharmacology. Rockville, 1976.
Hedlund 2004
-
- Hedlund PB, Sutcliffe JG. Functional, molecular and pharmacological advances in 5‐HT7 receptor research. Trends in Pharmacological Sciences 2004;25:481‐6. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.. Available from www.cochrane‐handbook.org.
Hutton 2009
-
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986.
Kay 1987
-
- Kay SR, Fiszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13:261‐76. - PubMed
Kohler 2007
Kumar 2012
-
- Kumar A, Narayan M, Raja H, Mathen Manoj J. Asenapine versus typical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010230; CD010230] - DOI
Lankappa 2012
-
- Lankappa S, Gandhi R. Quetiapine versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009935; CD009935] - DOI
Lehmann 1997
-
- Lehmann HE, Ban TA. The history of the psychopharmacology of schizophrenia. Canadian Journal of Psychiatry 1997;42:152‐62. - PubMed
Leon 2006
-
- Leon AC, Mallinckrodt CH, Chuang‐Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59(11):1001‐5. [PUBMED: 16905632] - PubMed
Leucht 2005
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] - PubMed
Leucht 2013
-
- Leucht S, Zhao J. Early improvement as a predictor of treatment response and remission in patients with acute schizophrenia: effects of asenapine. European Neuropsychopharmacology 2013; Vol. 23:S470.
Leucht 2014
-
- Leucht S, Zhao J. Early improvement as a predictor of treatment response and remission in patients with schizophrenia: a pooled, post‐hoc analysis from the asenapine development program. Journal of Psychopharmacology 2014;28:387‐94. - PubMed
Liddle 1987
-
- Liddle PF. The symptoms of chronic schizophrenia. A re‐examination of the positive‐negative dichotomy. British Journal of Psychiatry 1987;151:145‐51. - PubMed
Lieberman 2004
Marder 1997
-
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: Combined results of the North American trials. Journal of Clinical Psychiatry 1997;58:538‐46. - PubMed
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
McGrath 2008
-
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiological Reviews 2008;30:67‐76. - PubMed
Meltzer 1999
-
- Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 1999;21:106S‐15S. - PubMed
Moher 2010
Muench 2010
-
- Muench J, Hamer AN. Adverse effects of antipsychotic medications. American Family Physician 2010;81:617‐22. - PubMed
Munetz 1988
-
- Munetz MR, Benjamin S. How to examine patients using the Abnormal Involuntary Movement Scale. Hospital & Community Psychiatry 1988;39:1172‐7. - PubMed
Nicholson 1983
-
- Nicholson AN. Antihistamines and sedation. The Lancet 1983;322:211‐2. - PubMed
Overall 1962
-
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812.
Preda 2010
-
- Preda A, Faziola L. Asenapine versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008902; CD008902] - DOI
Rattehalli 2010
Saha 2005
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011.
Shahid 2009
-
- Shahid M, Walker GB, Zorn SH, Wong E. Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. Journal of Psychopharmacology 2009;23:65‐73. - PubMed
Simpson 1970
-
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 1970;45:11‐9. - PubMed
Siris 2000
-
- Siris SG. Depression in schizophrenia: perspective in the era of "atypical" antipsychotic agents. American Journal of Psychiatry 2000;157:1379‐89. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stoner 2012
-
- Stoner SC, Pace HA. Asenapine: a clinical review of a second‐generation antipsychotic. Clinical Therapeutics 2012;34:1023‐40. - PubMed
Storosum 1998
-
- Storosum JG, Elferink AJA, Zwieten BJ. Schizophrenia: do we really need placebo‐controlled studies?. European Neuropsychopharmacology 1998;8:279‐86. - PubMed
Svensson 2003
-
- Svensson TH. a‐Adrenoceptor modulation hypothesis of antipsychotic atypicality. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2003;27:1145‐58. - PubMed
Sycrest 2014
-
- Sycrest. Sycrest patient information leaflet. http://www.sycrest.co.uk/siteuploads/PIL.pdf 2014.
Szegedi 2012
-
- Szegedi A, Verweij P, Duijnhoven W, Mackle M, Cazorla P, Fennema H. Meta‐analyses of the efficacy of asenapine for acute schizophrenia: Comparisons with placebo and other antipsychotics. Journal of Clinical Psychiatry 2012;73:1533‐40. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. - PubMed
van Os 2008
Walker 2004
-
- Walker E, Kestler L, Bollini A, Hochman KM. Schizophrenia: etiology and course. Annual Review of Psychology 2004;55:401‐30. - PubMed
WHO 2014
-
- World Health Organization. Schizophrenia. http://www.who.int/mental_health/management/schizophrenia/en/ 2014.
Worrel 2000
-
- Worrel JA, Marken PA, Beckman SE, Ruehter VL. Atypical antipsychotic agents: a critical review. American Journal of Health‐System Pharmacy 2000;57:238‐55. - PubMed
Xia 2009
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

