Planned early delivery versus expectant management of the term suspected compromised baby for improving outcomes
- PMID: 26599471
- PMCID: PMC8935540
- DOI: 10.1002/14651858.CD009433.pub2
Planned early delivery versus expectant management of the term suspected compromised baby for improving outcomes
Abstract
Background: Fetal compromise in the term pregnancy is suspected when the following clinical indicators are present: intrauterine growth restriction (IUGR), decreased fetal movement (DFM), or when investigations such as cardiotocography (CTG) and ultrasound reveal results inconsistent with standard measurements. Pathological results would necessitate the need for immediate delivery, but the management for 'suspicious' results remains unclear and varies widely across clinical centres. There is clinical uncertainty as to how to best manage women presenting with a suspected term compromised baby in an otherwise healthy pregnancy.
Objectives: To assess, using the best available evidence, the effects of immediate delivery versus expectant management of the term suspected compromised baby on neonatal, maternal and long-term outcomes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 May 2015) and reference lists of retrieved studies.
Selection criteria: Randomised or quasi-randomised controlled trials comparing expectant management versus planned early delivery for women with a suspected compromised fetus from 37 weeks' gestation or more.
Data collection and analysis: Two review authors independently assessed trials for inclusion and assessed trial quality. Two review authors independently extracted data. Data were checked for accuracy. We assessed the quality of the evidence using the GRADE approach.
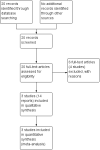
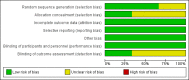
Main results: Of the 20 reports identified by the search strategy, we included three trials (546 participants: 269 to early delivery and 277 to expectant management), which met our inclusion criteria. Two of the trials compared outcomes in 492 pregnancies with IUGR of the fetus, and one in 54 pregnancies with oligohydramnios. All three trials were of reasonable quality and at low risk of bias. The level of evidence was graded moderate, low or very low, downgrading mostly for imprecision and for some indirectness. Overall, there was no difference in the primary neonatal outcomes of perinatal mortality (no deaths in either group, one trial, 459 women, evidence graded moderate), major neonatal morbidity (risk ratio (RR) 0.15, 95% confidence interval (CI) 0.01 to 2.81, one trial, 459 women, evidence graded low), or neurodevelopmental disability/impairment at two years of age (RR 2.04, 95% CI 0.62 to 6.69,one trial, 459 women, evidence graded low). There was no difference in the risk of necrotising enterocolitis (one trial, 333 infants) or meconium aspiration (one trial, 459 infants), There was also no difference in the reported primary maternal outcomes: maternal mortality (RR 3.07, 95% CI 0.13 to 74.87, one trial, 459 women, evidence graded low), and significant maternal morbidity (RR 0.92, 95% CI 0.38 to 2.22, one trial, 459 women, evidence graded low).The gestational age at birth was on average 10 days earlier in women randomised to early delivery (mean difference (MD) -9.50, 95% CI -10.82 to -8.18, one trial, 459 women) and women in the early delivery group were significantly less likely to have a baby beyond 40 weeks' gestation (RR 0.10, 95% CI 0.01 to 0.67, one trial, 33 women). Significantly more infants in the planned early delivery group were admitted to intermediate care nursery (RR 1.28, 95% CI 1.02 to 1.61, two trials, 491 infants). There was no difference in the risk of respiratory distress syndrome, (one trial, 333 infants), Apgar score less than seven at five minutes (three trials, 546 infants), resuscitation required (one trial, 459 infants), mechanical ventilation (one trial, 337 infants), admission to neonatal intensive care unit (NICU) (RR 0.88, 95% CI 0.35 to 2.23, three trials, 545 infants, evidence graded very low), length of stay in NICU/SCN (one trial, 459 infants), and sepsis (two trials, 366 infants).Babies in the expectant management group were more likely to be < 2.3rd centile for birthweight (RR 0.51, 95% CI 0.36 to 0.73, two trials, 491 infants), however there was no difference in the proportion of babies with birthweight < 10th centile (RR 0.98, 95% CI 0.88 to 1.10). There was no difference in any of the reported maternal secondary outcomes including: caesarean section rates (RR 1.02, 95% CI 0.65 to 1.59, three trials, 546 women, evidence graded low), placental abruption (one trial, 459 women), pre-eclampsia (one trial, 459 women), vaginal birth (three trials 546 women), assisted vaginal birth (three trials 546 women), breastfeeding rates (one trial, 218 women), and number of weeks of breastfeeding after delivery one trial, 124 women). There was an expected increase in induction in the early delivery group (RR 2.05, 95% CI 1.78 to 2.37, one trial, 459 women).No data were reported for the pre-specified secondary neonatal outcomes of the number of days of mechanical ventilation, moderate-severe hypoxic ischaemic encephalopathy or need for therapeutic hypothermia. Likewise, no data were reported for secondary maternal outcomes of postnatal infection, maternal satisfaction or views of care.
Authors' conclusions: A policy for planned early delivery versus expectant management for a suspected compromised fetus at term does not demonstrate any differences in major outcomes of perinatal mortality, significant neonatal or maternal morbidity or neurodevelopmental disability. In women randomised to planned early delivery, the gestational age at birth was on average 10 days earlier, women were less likely to have a baby beyond 40 weeks' gestation, they were more likely to be induced and infants were more likely to be admitted to intermediate care nursery. There was also a significant difference in the proportion of babies with a birthweight centile < 2.3rd, however this did not translate into a reduction in morbidity. The review is informed by only one large trial and two smaller trials assessing fetuses with IUGR or oligohydramnios and therefore cannot be generalised to all term pregnancies with suspected fetal compromise. There are other indications for suspecting compromise in a fetus at or near term such as maternal perception of DFM, and ultrasound and/or CTG abnormalities. Future randomised trials need to assess effectiveness of timing of delivery for these indications.
Conflict of interest statement
Diana Bond: We are grateful to the Stillbirth Foundation Australia for their generous funding to support the Sydney Stillbirth Study. SFA has had no input or influence regarding the publication of this review.
Angela Carberry: None known.
Adrienne Gordon: Diana bond is supported for her work on another project by a grant from the stillbirth foundation Australia. The charity was not involved with this review and we do not believe there is a conflict of interest.
Jon Hyett: None known.
Jonathan Morris: None known.
Bradley de Vries: I am employed by Royal Prince Alfred Hospital as a staff specialist obstetrician.
Figures






























Update of
- doi: 10.1002/14651858.CD009433
References
References to studies included in this review
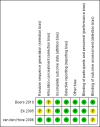
Boers 2010 {published data only}
-
- Boers K, Vijgen S, Bijlenga D, Post J, Bekedam D, Kwee A, et al. Induction of labour versus expectant monitoring for intrauterine growth restriction at term (The Digitat Trial): a multicentre randomised controlled trial. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S3.
-
- Boers KE, Wyk L, Post JA, Kwee A, Pampus MG, Spaanderdam ME, et al. Neonatal morbidity after induction vs expectant monitoring in intrauterine growth restriction at term: a subanalysis of the DIGITAT RCT. American Journal of Obstetrics & Gynecology 2012;206(4):344.e1‐7. - PubMed
Ek 2005 {published data only}
-
- Ek S, Andersson A, Johansson A, Kublicas M. Oligohydramnios in uncomplicated pregnancies beyond 40 completed weeks A prospective, randomised, pilot study on maternal and neonatal outcomes. Fetal Diagnosis and Therapy 2005;20(3):182‐5. - PubMed
van den Hove 2006 {published data only}
-
- Hove MM, Willekes C, Roumen FJ, Scherjon SA. Intrauterine growth restriction at term: induction or spontaneous labour? Disproportionate intrauterine growth intervention trial at term (DIGITAT): a pilot study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;125(1):54‐8. - PubMed
References to studies excluded from this review
Conway 2000 {published data only}
-
- Conway DL, Groth S, Adkins WB, Langer O. Management of isolated oligohydramnios in the term pregnancy: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S21.
Dogra 2012 {published data only}
-
- Dogra Y. Elective induction vs spontaneous labour in patients with heart disease. ClinicalTrials.gov 2012.
Nicholson 2008 {published data only}
-
- Nicholson J, Caughey A, Parry S, Rosen S, Evans A, Macones G. Prospective randomized trial of the active management of risk in pregnancy at term: improved birth outcomes from prostaglandin‐assisted preventive labor induction. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S37, Abstract no: 84.
-
- Peek MJ, Nanan RK. The role of sepsis in the AMOR‐IPAT Trial... (Am J Obstet Gynecol. 2008 May;198(5):511.e1‐15). American Journal of Obstetrics & Gynecology 2009;200(4):e12. - PubMed
Pri‐Paz 2008 {published data only}
-
- Pri‐Paz SM. Active management of risk in pregnancy at term to reduce rate of cesarean deliveries (AMOR IPAT). ClinicalTrials.gov (http://clinicaltrials.gov) accessed 19 February 2008.
Additional references
Alfirevic 2013
ANZSA 2010
-
- Preston S, Mahomed K, Chadha Y, Flenady V, Gardener G, MacPhail J, et al. Clinical Practice Guideline for the Management of Women Who Report Decreased Fetal Movements. Australia and New Zealand Stillbirth Alliance (ANZSA), July 2010.
Bijlenga 2011
Boers 2007
Boers 2009
-
- Boers K, Vijgen S, Bijlenga D, Post J, Bekedam D, Kwee A, et al. Induction of labour versus expectant monitoring for intrauterine growth restriction at term (The Digitat Trial): a multicentre randomised controlled trial. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S3.
Boers 2012
-
- Boers KE, Wyk L, Post JA, Kwee A, Pampus MG, Spaanderdam ME, et al. Neonatal morbidity after induction vs expectant monitoring in intrauterine growth restriction at term: a subanalysis of the DIGITAT RCT. American Journal of Obstetrics & Gynecology 2012;206(4):344.e1‐7. - PubMed
Boulvain 2001
Brown 1981
-
- Brown R, Patrick J. The nonstress test: how long is enough?. American Journal of Obstetrics & Gynecology 1981;141(6):646‐51. - PubMed
Chauhan 2012
-
- Chauhan S, Tajik P, Boers K, Wyk L, Mol B, Scherjon S. Differentiating newborns with birth weight < vs > 3 percentiles for gestational age: secondary‐analysis of a randomized clinical trial (DIGITAT). American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S177‐S178.
Cheng 2008
-
- Cheng YW, Nicholson JM, Nakagawa S, Bruckner TA, Washington AE, Caughey AB. Perinatal outcomes in low‐risk term pregnancies: do they differ by week of gestation?. American Journal of Obstetrics and Gynecology 2008;199:370.e1‐370.e7. - PubMed
Evertson 1979
-
- Evertson LR, Gauthier RJ, Schifrin BS, Paul RH. Antepartum fetal heart rate testing. I. Evolution of the nonstress test. American Journal of Obstetrics and Gynecology 1979;133(1):29‐33. - PubMed
Froen 2004
-
- Froen JF. A kick from within ‐ fetal movement counting and the cancelled progress in antenatal care. Journal of Perinatal Medicine 2004;32:13‐24. - PubMed
Froen 2008
-
- Froen JF, Tveit JV, Saastad E, Bordahl PE, Stray‐Pedersen B, Heazell AE, et al. Management of decreased fetal movements. Seminars in Perinatology 2008;32(4):307‐11. - PubMed
Grivell 2015
Gülmezoglu 2012
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2012
Irion 1998
Kean 1996
-
- Kean LH, Liu DT. Antenatal care as a screening tool for the detection of small for gestational age babies in the low risk population. Journal of Obstetrics and Gynaecology 1996;16(2):77‐82.
Maulik 2004
-
- Maulik D, Sicuranza G, Lysikiewicz A, Figueroa R. Fetal growth restriction: 3 keys to successful management. OBG Management 2004;16(6):48‐64.
Maulik 2006
-
- Maulik D. Management of fetal growth restriction: an evidence‐based approach. Clinical Obstetrics and Gynecology 2006;49(2):320‐34. - PubMed
Moore 1997
-
- Moore TR. Clinical assessment of amniotic fluid. Clinical Obstetrics and Gynecology 1997;40:303‐13. - PubMed
Morrison 1995
-
- Morrison JJ, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. British Journal of Obstetrics and Gynaecology 1995;102:101‐6. - PubMed
NICE/RCOG 2007
-
- National Collaborating Centre for Women's and Children's Health. Intrapartum care: Care of Healthy Women and their Babies During Childbirth. NICE Clinical Guideline 55. London: NICE, 2007 September.
Nicholson 2007
-
- Nicholson J, Caughey A, Parry S, Rosen S, Evans A, Macones G. Prospective randomized trial of the active management of risk in pregnancy at term: improved birth outcomes from prostaglandin‐assisted preventive labor induction. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S37, Abstract no: 84.
Novikova 2011
Peek 2009
-
- Peek MJ, Nanan RK. The role of sepsis in the AMOR‐IPAT Trial. American Journal of Obstetrics & Gynecology 2009;200(4):e12. - PubMed
RCOG 2011
-
- Whitworth MK, Fisher M, Heazell A. Reduced Fetal Movements. Greentop Guideline 57. Royal College of Obstetricians and Gynaecologists, February 2011.
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Seyb 1999
-
- Seyb ST, Berka RJ, Socol ML, Dooley SL. Risk of cesarean delivery with elective induction of labor at term in nulliparous women. Obstetrics & Gynecology 1999;94(4):600‐7. - PubMed
Smith 1992
-
- Smith CV, Plambeck RD, Rayburn WF, Albaugh KJ. Relation of mild idiopathic polyhydramnios to perinatal outcome. Obstetrics & Gynecology 1992;79(3):387‐9. - PubMed
Stock 2012
Stutchfield 2005
Tajik 2012
-
- Tajik P, Boers K, Wyk L, Mol B, Sicco S. Evaluation of markers guiding management decision for intrauterine growth restriction: a sub‐analysis of a randomized trial, DIGITAT. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S199‐200.
Tajik 2014
-
- Tajik P, Wyk L, Boers KE, Cessie S, Zafarmand MH, Roumen F, et al. Which intrauterine growth restricted fetuses at term benefit from early labour induction? A secondary analysis of the DIGITAT randomised trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2014;172:20‐5. - PubMed
Van Wyk 2012
-
- Wyk L. Effects on (neuro) developmental and behavioral outcome at 2 years of age of induced labor compared with expectant management in intra‐uterine growth restricted infants: long‐term outcomes of the DIGITAT‐trial. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S19‐20. - PubMed
Van Wyk 2012a
-
- Wyk L, Boers KE, Post JAM, Pampus MG, Wassenaer AG, Baar AL, et al. DIGITAT Study Group. Effects on (neuro)developmental and behavioral outcome at 2 years of age of induced labor compared with expectant management in intrauterine growth‐restricted infants: Long‐term outcomes of the DIGITAT trial. American Journal of Obstetrics and Gynecology 2012;206(5):406.e1‐406.e7. - PubMed
Vijgen 2013
-
- Vijgen SM, Boers KE, Opmeer BC, Bijlenga D, Bekedam DJ, Bloemenkamp KW, et al. Economic analysis comparing induction of labour and expectant management for intrauterine growth restriction at term (DIGITAT trial). European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;170(2):358‐63. - PubMed
Visser 2009
-
- Visser GHA, Eilers PHC, Elferink‐Stinkens PM, Merkus HM WM, Wit JM. New Dutch reference curves for birthweight by gestational age. Early Human Development 2009;85(12):737‐44. - PubMed
Willekes 2011
-
- Willekes C, Post J, Mol BW, Roumen F, Boers K, Delemarre F, et al. Neonatal morbidity after induction vs expectant monitoring in at term growth restriction (DIGITAT trial). American Journal of Obstetrics and Gynecology 2011;204(1 Suppl 1):S71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

