Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD)
- PMID: 26599576
- PMCID: PMC8763351
- DOI: 10.1002/14651858.CD009885.pub2
Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD)
Update in
-
Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD).Cochrane Database Syst Rev. 2023 Mar 27;3(3):CD009885. doi: 10.1002/14651858.CD009885.pub3. Cochrane Database Syst Rev. 2023. Update in: Cochrane Database Syst Rev. 2025 Dec 4;12:CD009885. doi: 10.1002/14651858.CD009885.pub4. PMID: 36971690 Free PMC article. Updated.
Abstract
Background: Attention deficit hyperactivity disorder (ADHD) is one of the most commonly diagnosed and treated psychiatric disorders in childhood. Typically, children with ADHD find it difficult to pay attention, they are hyperactive and impulsive.Methylphenidate is the drug most often prescribed to treat children and adolescents with ADHD but, despite its widespread use, this is the first comprehensive systematic review of its benefits and harms.
Objectives: To assess the beneficial and harmful effects of methylphenidate for children and adolescents with ADHD.
Search methods: In February 2015 we searched six databases (CENTRAL, Ovid MEDLINE, EMBASE, CINAHL, PsycINFO, Conference Proceedings Citations Index), and two trials registers. We checked for additional trials in the reference lists of relevant reviews and included trials. We contacted the pharmaceutical companies that manufacture methylphenidate to request published and unpublished data.
Selection criteria: We included all randomised controlled trials (RCTs) comparing methylphenidate versus placebo or no intervention in children and adolescents aged 18 years and younger with a diagnosis of ADHD. At least 75% of participants needed to have an intellectual quotient of at least 70 (i.e. normal intellectual functioning). Outcomes assessed included ADHD symptoms, serious adverse events, non-serious adverse events, general behaviour and quality of life.
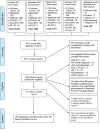
Data collection and analysis: Seventeen review authors participated in data extraction and risk of bias assessment, and two review authors independently performed all tasks. We used standard methodological procedures expected within Cochrane. Data from parallel-group trials and first period data from cross-over trials formed the basis of our primary analyses; separate analyses were undertaken using post-cross-over data from cross-over trials. We used Trial Sequential Analyses to control for type I (5%) and type II (20%) errors, and we assessed and downgraded evidence according to the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach for high risk of bias, imprecision, indirectness, heterogeneity and publication bias.
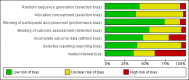
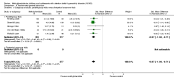
Main results: The studies.We included 38 parallel-group trials (5111 participants randomised) and 147 cross-over trials (7134 participants randomised). Participants included individuals of both sexes, at a boys-to-girls ratio of 5:1, and participants' ages ranged from 3 to 18 years across most studies (in two studies ages ranged from 3 to 21 years). The average age across all studies was 9.7 years. Most participants were from high-income countries.The duration of methylphenidate treatment ranged from 1 to 425 days, with an average duration of 75 days. Methylphenidate was compared to placebo (175 trials) or no intervention (10 trials). Risk of Bias.All 185 trials were assessed to be at high risk of bias. Primary outcomes. Methylphenidate may improve teacher-rated ADHD symptoms (standardised mean difference (SMD) -0.77, 95% confidence interval (CI) -0.90 to -0.64; 19 trials, 1698 participants; very low-quality evidence). This corresponds to a mean difference (MD) of -9.6 points (95% CI -13.75 to -6.38) on the ADHD Rating Scale (ADHD-RS; range 0 to 72 points; DuPaul 1991a). A change of 6.6 points on the ADHD-RS is considered clinically to represent the minimal relevant difference. There was no evidence that methylphenidate was associated with an increase in serious (e.g. life threatening) adverse events (risk ratio (RR) 0.98, 95% CI 0.44 to 2.22; 9 trials, 1532 participants; very low-quality evidence). The Trial Sequential Analysis-adjusted intervention effect was RR 0.91 (CI 0.02 to 33.2).
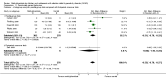
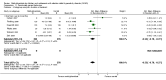
Secondary outcomes: Among those prescribed methylphenidate, 526 per 1000 (range 448 to 615) experienced non-serious adverse events, compared with 408 per 1000 in the control group. This equates to a 29% increase in the overall risk of any non-serious adverse events (RR 1.29, 95% CI 1.10 to 1.51; 21 trials, 3132 participants; very low-quality evidence). The Trial Sequential Analysis-adjusted intervention effect was RR 1.29 (CI 1.06 to 1.56). The most common non-serious adverse events were sleep problems and decreased appetite. Children in the methylphenidate group were at 60% greater risk for trouble sleeping/sleep problems (RR 1.60, 95% CI 1.15 to 2.23; 13 trials, 2416 participants), and 266% greater risk for decreased appetite (RR 3.66, 95% CI 2.56 to 5.23; 16 trials, 2962 participants) than children in the control group.Teacher-rated general behaviour seemed to improve with methylphenidate (SMD -0.87, 95% CI -1.04 to -0.71; 5 trials, 668 participants; very low-quality evidence).A change of seven points on the Child Health Questionnaire (CHQ; range 0 to 100 points; Landgraf 1998) has been deemed a minimal clinically relevant difference. The change reported in a meta-analysis of three trials corresponds to a MD of 8.0 points (95% CI 5.49 to 10.46) on the CHQ, which suggests that methylphenidate may improve parent-reported quality of life (SMD 0.61, 95% CI 0.42 to 0.80; 3 trials, 514 participants; very low-quality evidence).
Authors' conclusions: The results of meta-analyses suggest that methylphenidate may improve teacher-reported ADHD symptoms, teacher-reported general behaviour, and parent-reported quality of life among children and adolescents diagnosed with ADHD. However, the low quality of the underpinning evidence means that we cannot be certain of the magnitude of the effects. Within the short follow-up periods typical of the included trials, there is some evidence that methylphenidate is associated with increased risk of non-serious adverse events, such as sleep problems and decreased appetite, but no evidence that it increases risk of serious adverse events.Better designed trials are needed to assess the benefits of methylphenidate. Given the frequency of non-serious adverse events associated with methylphenidate, the particular difficulties for blinding of participants and outcome assessors point to the advantage of large, 'nocebo tablet' controlled trials. These use a placebo-like substance that causes adverse events in the control arm that are comparable to those associated with methylphenidate. However, for ethical reasons, such trials should first be conducted with adults, who can give their informed consent.Future trials should publish depersonalised individual participant data and report all outcomes, including adverse events. This will enable researchers conducting systematic reviews to assess differences between intervention effects according to age, sex, comorbidity, type of ADHD and dose. Finally, the findings highlight the urgent need for large RCTs of non-pharmacological treatments.
Conflict of interest statement
Ole Jakob Storebø ‐ none known. Erica Ramstad ‐ none known. Helle B. Krogh ‐ none known. Trine Danvad Nilausen ‐ none known. Maria Skoog ‐ none known. Mathilde Holmskov ‐ none known. Susanne Rosendal ‐ none known. Camilla Groth ‐ received funds from the Lundbeck Foundation to finance part of her Ph.D in the paediatric field on Tourette Syndrome. CG confirms that none of these funds were used to work on this review. Frederik Løgstrup Magnusson ‐ none known. Carlos R Moreira‐Maia ‐ receives financial research support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). CRMM has served as speaker for Novartis, has developed educational materials for Novartis, has received travel awards from the Health Technology Assessment Institute (IATS) and the Universidade Federal do Rio Grande do Sul (UFRGS), and has received travel and registration support to attend the 5th World Congress on ADHD from the World Federation of ADHD. Donna Gillies ‐ is an Editor with the Cochrane Developmental, Psychosocial and Learning Problems Group. Kirsten Buch Rasmussen ‐ none known. Dorothy Gauci ‐ none known. Morris Zwi ‐ sits on the Paediatric Medicines Expert Advisory Group at the Medicines and Healthcare Products Regulatory Agency, which considers applications regarding licensing of paediatric medicines. Payment for MZ's attendance at this meeting goes to his National Health Service (NHS) organisation. Richard Kirubakaran ‐ is currently employed by Cochrane South Asia. His salary is funded by the Effective Healthcare Research Consortium (EHCRC) for the Department for International Development (DFID), UK. Bente Forsbøl ‐ none known. Erik Simonsen ‐ none known. Christian Gluud ‐ none known.
Figures

















































































Comment in
-
Methylphenidate for attention-deficit/hyperactivity disorder: Too much of a good thing?Aust N Z J Psychiatry. 2016 Feb;50(2):113-4. doi: 10.1177/0004867415626823. Aust N Z J Psychiatry. 2016. PMID: 26823533 No abstract available.
-
The evidence base of methylphenidate for children and adolescents with attention-deficit hyperactivity disorder is in fact flawed.Eur Child Adolesc Psychiatry. 2016 Sep;25(9):1037-8. doi: 10.1007/s00787-016-0855-0. Epub 2016 Apr 30. Eur Child Adolesc Psychiatry. 2016. PMID: 27138912 No abstract available.
-
Methylphenidate for Attention-Deficit/Hyperactivity Disorder in Children and Adolescents.JAMA. 2016 May 10;315(18):2009-10. doi: 10.1001/jama.2016.3611. JAMA. 2016. PMID: 27163989
-
Trust, but verify. The errors and misinterpretations in the Cochrane analysis by O. J. Storebo and colleagues on the efficacy and safety of methylphenidate for the treatment of children and adolescents with ADHD.Z Kinder Jugendpsychiatr Psychother. 2016;44(4):307-14. doi: 10.1024/1422-4917/a000433. Epub 2016 Jun 6. Z Kinder Jugendpsychiatr Psychother. 2016. PMID: 27270192
-
Check and Double Check - the Cochrane review by Storebo et al. (2015) is indeed flawed.Z Kinder Jugendpsychiatr Psychother. 2016 Sep;44(5):336-337. doi: 10.1024/1422-4917/a000473. Z Kinder Jugendpsychiatr Psychother. 2016. PMID: 27658628 No abstract available.
-
Methylphenidate for ADHD in children and adolescents: throwing the baby out with the bathwater.Evid Based Ment Health. 2016 Nov;19(4):97-99. doi: 10.1136/eb-2016-102461. Epub 2016 Oct 6. Evid Based Ment Health. 2016. PMID: 27935807 Free PMC article.
References
References to studies included in this review
Abikoff 2009 {published data only}
-
- Concerta for organizational deficits in ADHD. The Brown University Child and Adolescent Behavior Letter 2009; Vol. 25, issue 3:2.
-
- Abikoff H, Nissley‐Tsiopinis J, Gallagher R, Zambenedetti M, Seyffert M, Boorady R, et al. Effects of MPH‐OROS on the organizational, time management, and planning behaviors of children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(2):166‐75. - PubMed
Ahmann 1993 {published data only}
-
- Ahmann PA, Waltonen SJ, Olson KA, Theye FW, Erem AJ, LaPlant RJ. Placebo‐controlled evaluation of Ritalin side effects. Pediatrics 1993;91(6):1101‐6. - PubMed
Armstrong 2012 {published data only}
-
- Significant effect of OROS MPH ER on attention. Brown University Child & Adolescent Psychopharmacology Update 2010; Vol. 12, issue 1:7‐8.
-
- Armstrong RB, Damaraju CV, Ascher S, Schwarzman L, O'Neill J, Starr HL. Time course of treatment effect of OROS® methylphenidate in children with ADHD. Journal of Attention Disorders 2012;16(8):697‐705. - PubMed
Arnold 2004 {published data only}
-
- Arnold LE, Lindsay RL, Conners CK, Wigal SB, Levine AJ, Johnson DE, et al. A double‐blind, placebo‐controlled withdrawal trial of dexmethylphenidate hydrochloride in children with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2004; Vol. 14, issue 4:542‐54. - PubMed
Ashare 2010 {published data only}
Barkley 1989 {published data only}
-
- Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo‐controlled evaluation. Pediatrics 1990;86(2):184‐92. - PubMed
-
- Barkley RA, McMurray MB, Edelbrock CS, Robbins K. The response of aggressive and nonaggressive ADHD children to two doses of methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(6):873‐81. - PubMed
Barkley 1991 {published data only}
-
- Barkley RA, DuPaul GJ, McMurray MB. Attention deficit disorder with and without hyperactivity: clinical response to three dose levels of methylphenidate. Pediatrics 1991;87(4):519‐31. - PubMed
-
- DuPaul GJ, Barkley RA, McMurray MB. Response of children with ADHD to methylphenidate: interaction with internalizing symptoms. Journal of the American Academy of Child and Adolescent Psychiatry 1994;33(6):894‐903. - PubMed
Barkley 2000 {published data only}
-
- Barkley RA, Connor DF, Kwasnik D. Challenges to determining adolescent medication response in an outpatient clinical setting: comparing Adderall and methylphenidate for ADHD. Journal of Attention Disorders 2000;4(2):102‐13.
Bedard 2008 {published data only}
-
- Bedard A‐C, Tannock R. Anxiety, methylphenidate response, and working memory in children with ADHD. Journal of Attention Disorders 2008;11(5):546‐57. - PubMed
Ben 2002 {published data only}
-
- Ben AL, Grizenko N, Mbekou V, Lageix P, Baron C, Schwartz G, et al. Dopamine transporter gene and behavioral response to methylphenidate (MPH) in children with attention deficit hyperactivity disorder (ADHD). American Journal of Medical Genetics. Abstracts of the Xth World Congress of Psychiatric Genetics; 2002 October 9‐13; Brussels, Belgium. 2002; Vol. 114 (7):724‐5.
Biederman 2003 {published data only}
-
- Biederman J, Quinn D, Weiss M, Markabi S, Weidenman M, Edson K, et al. Efficacy and safety of Ritalin LA, a new, once daily, extended‐release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatric Drugs 2003;5(12):833‐41. - PubMed
Bliznakova 2007 {published data only}
-
- Bliznakova L, Gerstner S, Schmidt MH, Becker K. Methylphenidate double‐blind trial: indication and performing [Der methylphenidat‐doppelblindversuch ‐ indikation und durchfuhrung]. Klinische Pädiatrie 2007;219(1):9‐16. - PubMed
Blum 2011 {published data only}
-
- Blum NJ, Jawad AF, Clarke AT, Power TJ. Effect of osmotic‐release oral system methylphenidate on different domains of attention and executive functioning in children with attention‐deficit‐hyperactivity disorder. Developmental Medicine & Child Neurology 2011;53(9):843‐9. - PubMed
Borcherding 1990 {published data only}
-
- Borcherding BG, Keysor CS, Rapoport JL, Elia J, Amass J. Motor/vocal tics and compulsive behaviors on stimulant drugs: is there a common vulnerability?. Psychiatry Research 1990;33(1):83‐94. - PubMed
Brams 2008 {published data only}
-
- Brams M, Muniz R, Childress A, Giblin J, Mao A, Turnbow J, et al. A randomized, double‐blind, crossover study of once‐daily dexmethylphenidate in children with attention‐deficit hyperactivity disorder: rapid onset of effect. CNS Drugs 2008;22(8):693‐704. - PubMed
Brams 2012 {published data only}
-
- Dexmethylphenidate may be effective later in the day. The Brown University Child & Adolescent Psychopharmacology Update 2012; Vol. 14, issue 11:8.
-
- Brams M, Turnbow J, Pestreich L. Erratum: a randomized, double‐blind study of 30 versus 20 mg dexmethylphenidate extended‐release in children with attention‐deficit/ hyperactivity disorder: late‐day symptom control. Journal of Clinical Psychopharmacology 2012;32(6):766. Erratum for: Journal of Clinical Psychopharmacology 2012;32(5):637‐44. - PubMed
-
- Brams M, Turnbow J, Pestreich L, Giblin J, Childress A, McCague K, et al. A randomized, double‐blind study of 30 versus 20 mg dexmethylphenidate extended‐release in children with attention‐deficit/hyperactivity disorder: late‐day symptom control. Journal of Clinical Psychopharmacology. M. Brams, Menninger Department of Psychiatry, Baylor College of Medicine, 500 Westcott St, Houston, TX 77007, United States. E‐mail: drmattbrams@aol.com: Lippincott Williams and Wilkins (530 Walnut Street,P O Box 327, Philadelphia PA 19106‐3621, United States), 2012; Vol. 32, issue 5:637‐44. - PubMed
-
- Muniz R, Pestreich L, McCague K, Padilla A, Brams M, Childress A. Extended‐release dexmethylphenidate 30 mg improves late‐day attention deficit hyperactivity disorder (ADHD) symptom control in children with ADHD: a randomized, double‐blind crossover study. Journal of Child and Adolescent Psychopharmacology. Abstracts of the 50th Annual National Institute of Mental Health (NIMH) New Clinical Drug Evaluation Unit (NCDEU) Meeting; 2010 June 14–17; Boca Raton, Florida 2010;20(6):534‐5.
-
- Padilla A, Pestreich L, McCague K, Muniz R. Late‐day attention deficit hyperactivity disorder (ADHD) symptom control improvement with extended‐release dexmethylphenidate in children with ADHD of all ethnicities: a sub‐analysis. Journal of Child and Adolescent Psychopharmacology. Abstracts of the 50th Annual National Institute of Mental Health (NIMH) New Clinical Drug Evaluation Unit (NCDEU) Meeting; 2010 June 14–17; Boca Raton, Florida 2010;20(6):534.
Brown 1984a {published data only}
-
- Brown RT, Wynne ME, Slimmer LW. Attention deficit disorder and the effect of methylphenidate on attention, behavioral, and cardiovascular functioning. Journal of Clinical Psychiatry 1984;45(11):473‐6. - PubMed
Brown 1985 {published data only}
-
- Brown RT, Wynne ME, Medenis R. Methylphenidate and cognitive therapy: a comparison of treatment approaches with hyperactive boys. Journal of Abnormal Child Psychology 1985;13(1):69‐87. - PubMed
Brown 1988 {published data only}
-
- Brown RT, Sexson SB. A controlled trial of methylphenidate in black adolescents. Attentional, behavioral, and physiological effects. Clinical Pediatrics 1988;27(2):74‐81. - PubMed
-
- Brown RT, Sexson SB. Effects of methylphenidate on cardiovascular responses in attention deficit hyperactivity disordered adolescents. Journal of Adolescent Health Care 1989;10(3):179‐83. - PubMed
Brown 1991 {published data only}
-
- Brown RT, Jaffe SL, Silverstein J, Magee H. Methylphenidate and hospitalized adolescents with conduct disorder: dose effects on classroom behavior, academic performance, and impulsivity. Journal of Youth and Adolescence 1991;20(5):501‐18. - PubMed
Buitelaar 1995 {published data only}
-
- Buitelaar JK, Swaab‐Barneveld H, Gaag RJ. Prediction of clinical response to methylphenidate in children with ADHD. X World Congress of Psychiatry; 1996 August 23‐26; Madrid, Spain. Madrid: World Psychiatric Association, 1996.
-
- Buitelaar JK, Gaag RJ, Swaab‐Barneveld H, Kuiper M. Pindolol and methylphenidate in children with attention‐deficit hyperactivity disorder. Clinical efficacy and side‐effects. Journal of Child Psychology and Psychiatry 1996;37(5):587‐95. - PubMed
-
- Buitelaar JK, Gaag RJ, Swaab‐Barneveld H, Kuiper M. Prediction of clinical response to methylphenidate in children with attention‐deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(8):1025‐32. - PubMed
Bukstein 1998 {published data only}
-
- Bukstein OG, Kolko DJ. Effects of methylphenidate on aggressive urban children with attention deficit hyperactivity disorder. Journal of Clinical Child Psychology 1998;27(3):340‐51. - PubMed
Butter 1983 {published data only}
-
- Butter HJ, Lapierre Y, Firestone P, Blank A. A comparative study of the efficacy of ACTH4‐9 analog, methylphenidate, and placebo on attention deficit disorder with hyperkinesis. Journal of Clinical Psychopharmacology 1983;3(4):226‐30. - PubMed
-
- Butter HJ, Lapierre Y, Firestone P, Blank A. Efficacy of ACTH 4‐9 analog, methylphenidate, and placebo on attention deficit disorder with hyperkinesis. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 1984;8(4‐6):661‐4. - PubMed
Carlson 1995 {published data only}
-
- Carlson GA, Rapport MD, Kelly KL, Pataki CS. Methylphenidate and desipramine in hospitalized children with comorbid behavior and mood disorders: separate and combined effects on behavior and mood. Journal of Child and Adolescent Psychopharmacology 1995;5(3):191‐204.
-
- Pataki CS, Carlson GA, Kelly KL, Rapport MD, Biancaniello TM. Side effects of methylphenidate and desipramine alone and in combination in children. Journal of the American Academy of Child and Adolescent Psychiatry 1993;32(5):1065‐72. - PubMed
Carlson 2007 {published data only}
Castellanos 1997 {published data only}
-
- Castellanos FX. Stimulants and tic disorders: from dogma to data. Archives of General Psychiatry 1999; Vol. 56, issue 4:337‐8. - PubMed
-
- Castellanos FX, Giedd JN, Elia J, Marsh WL, Ritchie GF, Hamburger SD, et al. Controlled stimulant treatment of ADHD and comorbid Tourette's syndrome: effects of stimulant and dose. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(5):589‐96. - PubMed
Chacko 2005 {published data only}
-
- Chacko A, Pelham WE, Gnagy EM, Greiner A, Vallano G, Bukstein O, et al. Stimulant medication effects in a summer treatment program among young children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2005;44(3):249‐57. - PubMed
Childress 2009 {published data only}
-
- Dose related safety and efficacy of dexmethylphenidate ER. The Brown University Child & Adolescent Psychopharmacology Update 2009; Vol. 11, issue 11:7‐8.
-
- Childress A, Muniz R, Miller J, Arnold V, Harper L, Gerstner O, et al. Fixed‐dose titration study of dexmethylphenidate extended release in children with ADHD: effects on teacher‐rated scales. Annals of Neurology 2007;62(Suppl 11):S125.
-
- Childress AC, Spencer T, Lopez F, Gerstner O, Thulasiraman A, Muniz R, et al. Efficacy and safety of dexmethylphenidate extended‐release capsules administered once daily to children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2009;19(4):351‐61. - PubMed
-
- Greenbaum M, Muniz R, Brams M, Boellner S, Gerstner O, Borello M, et al. Cardiovascular safety of dexmethylphenidate extended release in children with ADHD. Annals of Neurology 2007;62(Suppl 11):S146.
-
- Lopez F, Muniz R, Joyce JM, Franklin ER, Gerstner O, Borello M, et al. Fixed‐dose titration study of dexmethylphenidate extended release in children with ADHD: effects on parent‐rated scales. Annals of Neurology 2007;62(Suppl 11):S122.
Chronis 2003 {published data only}
-
- Chronis A, Pelham WE Jr, Gnagy E, Roberts J, Aronoff H. The impact of late‐afternoon stimulant dosing for children with ADHD on parent and parent‐child domains. Journal of Clinical Child and Adolescent Psychology 2003;32(1):118‐26. - PubMed
-
- Pelham WE, Gnagy EM, Chronis AM, Burrows‐MacLean L, Fabiano GA, Onyango AN, et al. A comparison of morning‐only and morning/late afternoon Adderall to morning‐only, twice‐daily, and three times‐daily methylphenidate in children with attention‐deficit/hyperactivity disorder. Pediatrics 1999;104(6):1300‐11. - PubMed
Coghill 2007 {published data only}
-
- Coghill DR, Rhodes SM, Matthews K. Chronic effects of the psychostimulant drug methylphenidate on neuropsychological functioning in drug‐naive boys with hyperkinetic disorder (ADHD). Journal of Psychopharmacology. Summer Meeting of the British Association for Psychopharmacology; 2003 July 20‐23; Cambridge, England. London: Sage Publications Ltd, 2003; Vol. 17 (3):A74. [WOS: 000185623500293]
-
- Coghill DR, Rhodes SM, Matthews K. The neuropsychological effects of chronic methylphenidate on drug‐naive boys with attention‐deficit/hyperactivity disorder. Biological Psychiatry 2007;62(9):954‐62. - PubMed
Coghill 2013 {published data only}
-
- Banaschewski T, Soutullo C, Lecendreux M, Johnson M, Zuddas A, Hodgkins P, et al. Health‐related quality of life and functional outcomes from a randomized, controlled study of lisdexamfetamine dimesylate in children and adolescents with attention deficit hyperactivity disorder. CNS Drugs 2013;27(10):829‐40. - PMC - PubMed
-
- Banaschewski T, Soutullo C, Lecendreux M, Johnson M, Zuddas A, Hodgkins P, et al. The child health and illness profile as a measure of health‐related quality of life in stimulant‐treated children and adolescents with ADHD. European Child & Adolescent Psychiatry. Abstracts of the 15th International Congress of ESCAP ‐ European Society for Child and Adolescent Psychiatry; 2013 July 6‐10; Dublin, Ireland 2013;22(2 Suppl):S125‐6.
-
- Coghill D, Banaschewski T, Lecendreux M, Soutollu C, Johnson M, Zuddas A, et al. The first European studies of lisdexamfetamine dimesylate in children and adolescents with attention‐deficit/hyperactivity disorder. European Child & Adolescent Psychiatry. Abstracts of the 15th International Congress of ESCAP ‐ European Society for Child and Adolescent Psychiatry; 2013 July 6‐10, Dublin, Ireland 2013;22(2 Suppl):S125.
-
- Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention‐deficit/hyperactivity disorder. European Neuropsychopharmacology 2013;23(10):1208‐18. - PubMed
-
- Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, et al. Post hoc comparison of the efficacy of lisdexamfetamine dimesylate and osmotic‐release oral system methylphenidate in children and adolescents with ADHD. European Psychiatry. Abstracts of the 21th European Congress of Psychiatry 2013;28(Suppl 1):Article 957.
Connor 2000 {published data only}
-
- Connor DF, Barkley RA, Davis HT. A pilot study of methylphenidate, clonidine, or the combination in ADHD comorbid with aggressive oppositional defiant or conduct disorder. Clinical Pediatrics 2000;39(1):15‐25. - PubMed
Cook 1993 {published data only}
-
- Cook JR. The Effects of Methylphenidate on Resource Allocation in the Mental Processing of ADD Children [PhD thesis]. University of North Dakota, 1989.
Corkum 2008 {published data only}
-
- Corkum P, Panton R, Ironside S, Macpherson M, Williams T. Acute impact of immediate release methylphenidate administered three times a day on sleep in children with attention‐deficit/hyperactivity disorder. Journal of Pediatric Psychology 2008;33(4):368‐79. - PubMed
-
- Ironside S, Davidson F, Corkum P. Circadian motor activity affected by stimulant medication in children with attention‐deficit/hyperactivity disorder. Journal of Sleep Research 2010;19(4):546‐51. - PubMed
Cox 2006 {published data only}
-
- Cox DJ, Merkel RL, Moore M, Thorndike F, Muller C, Kovatchev B. Relative benefits of stimulant therapy with OROS methylphenidate versus mixed amphetamine salts extended release in improving the driving performance of adolescent drivers with attention‐deficit/hyperactivity disorder. Pediatrics 2006;118(3):e704‐10. - PubMed
-
- Cox DJ, Moore M, Burket R, Merkel RL, Mikami AY, Kovatchev B. Rebound effects with long‐acting amphetamine or methylphenidate stimulant medication preparations among adolescent male drivers with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2008;18(1):1‐10. - PubMed
-
- Mikami AY, Cox DJ, Davis MT, Wilson HK, Merkel RL, Burket R. Sex differences in effectiveness of extended‐release stimulant medication among adolescents with attention‐deficit/hyperactivity disorder. Journal of Clinical Psychology in Medical Settings 2009;16(3):233‐42. - PubMed
-
- Wilson HK, Cox DJ, Merkel RL, Moore M, Coghill D. Effect of extended release stimulant‐based medications on neuropsychological functioning among adolescents with attention‐deficit/hyperactivity disorder. Archives of Clinical Neuropsychology 2006;21(8):797‐807. - PubMed
Döpfner 2004 {published data only}
-
- Döpfner M, Gerber WD, Banaschewski T, Breuer D, Freisleder FJ, Gerber‐von Müller G, et al. Comparative efficacy of once‐a‐day extended‐release methylphenidate, two‐times‐daily immediate‐release methylphenidate, and placebo in a laboratory school setting. European Child & Adolescent Psychiatry 2004;13(1 Suppl):i93‐101. - PubMed
-
- Döpfner M, Schröder S, Schmidt J, Lehmkuhl G. Duration of action of a single dose of methylphenidate Retard compared to twice immediate‐release methylphenidate in children and adolescents with ADHD [Wirkdauer einer einmaligen Gabe von Methylphenidat‐Retard im Vergleich zu zweimaliger Gabe von schnell freisetzendem Methylphenidat bei Kindern und Jugendlichen mit ADHS]. Klinikum der Universität zu Köln, Klinik und Poliklinik für Psychiatrie und Psychotherapie des Kindes und Jugendalters 2003.
-
- Gerber WD, Gerber‐von Müller G, Andrasik F, Niederberger U, Siniatchkin M, Kowalski JT, et al. The impact of a multimodal summer camp training on neuropsychological functioning in children and adolescents with ADHD: an exploratory study. Child Neuropsychology 2012;18(3):242‐55. - PubMed
-
- Gerber‐von Müller G, Petermann U, Petermann F, Niederberger U, Stephani U, Siniatchkin M, et al. ADHD summer camp: development and evaluation of a multimodal intervention program [Das ADHD‐summer‐camp‐entwicklung und evaluation eines multimodalen programms]. Kindheit und Entwicklung 2009;18(3):162‐72.
-
- Lehmkuhl G. Double‐blind, non‐inferiority trial investigating the duration of action of Medikinet‐retard vs. immediate‐release methylphenidate vs. placebo across the day in children with attention deficit hyperactivity disorder (ADHD). Integrated final report. Phase III. Universitätsklinikum Essen. Project number Medikinet‐retard (R) Trial 6520‐0073‐01 2005.
Douglas 1986 {published data only}
-
- Douglas VI, Barr RG, O'Neill ME, Britton BG. Short term effects of methylphenidate on the cognitive, learning and academic performance of children with attention deficit disorder in the laboratory and the classroom. Journal of Child Psychology and Psychiatry, and Allied Disciplines 1986;27(2):191‐211. - PubMed
Douglas 1995 {published data only}
-
- Douglas VI, Barr RG, Desilets J, Sherman E. Do high doses of stimulants impair flexible thinking in attention‐deficit hyperactivity disorder?. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(7):877‐85. - PubMed
DuPaul 1996 {published data only}
-
- DuPaul GJ, Anastopoulos AD, Kwasnik D, Barkley RA, McMurray MB, DuPaul GJ. Methylphenidate effects on children with attention deficit hyperactivity disorder: self‐report of symptoms, side‐effects, and self‐esteem. Journal of Attention Disorders 1996;1(1):3‐15.
Duric 2012 {published data only}
Epstein 2011 {published data only}
Fabiano 2007 {published data only}
-
- Fabiano GA, Pelham WE Jr, Gnagy EM, Burrows‐MacLean L, Coles EK, Chacko A, et al. The single and combined effects of multiple intensities of behavior modification and methylphenidate for children with attention deficit hyperactivity disorder in a classroom setting. School Psychology Review 2007; Vol. 36, issue 2:195‐216.
Findling 2006 {published data only}
-
- Findling RL, Quinn D, Hatch SJ, Cameron SJ, DeCory HH, McDowell M. Comparison of the clinical efficacy of twice‐daily Ritalin and once‐daily Equasym XL with placebo in children with attention deficit/hyperactivity disorder. European Child & Adolescent Psychiatry 2006;15(8):450‐9. - PubMed
Findling 2007 {published data only}
-
- Findling RL, Short EJ, McNamara NK, Demeter CA, Stansbrey RJ, Gracious BL, et al. Methylphenidate in the treatment of children and adolescents with bipolar disorder and attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(11):1445‐53. - PubMed
Findling 2008 {published data only}
-
- Faraone SV, Glatt SJ, Bukstein OG, Lopez FA, Arnold LE, Findling RL. Effects of once‐daily oral and transdermal methylphenidate on sleep behavior of children with ADHD. Journal of Attention Disorders 2009;12(4):308‐15. - PubMed
-
- Findling RL, Bukstein OG, Melmed RD, Lopez FA, Sallee FR, Arnold LE, et al. "A randomized, double‐blind, placebo‐controlled, parallel‐group study of methylphenidate transdermal system in pediatric patients with attention‐deficit/hyperactivity disorder": correction. Journal of Clinical Psychiatry 2008;69(2):329. - PubMed
-
- Findling RL, Bukstein OG, Melmed RD, López FA, Sallee FR, Arnold LE, et al. A randomized, double‐blind, placebo‐controlled, parallel‐group study of methylphenidate transdermal system in pediatric patients with attention‐deficit/hyperactivity disorder. Journal of Clinical Psychiatry 2008;69(1):149‐59. Erratum in: Journal of Clinical Psychiatry. 2008;69(2):329. - PubMed
-
- Swanson JM. Transdermal methylphenidate more effective than placebo for treating ADHD. Evidence‐Based Mental Health 2008;11(4):118. - PubMed
Findling 2010 {published data only}
-
- Findling RL, Katic A, Rubin R, Moon E, Civil R, Li Y. A 6‐month, open‐label, extension study of the tolerability and effectiveness of the methylphenidate transdermal system in adolescents diagnosed with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2010;20(5):365‐75. - PubMed
-
- Findling RL, Turnbow J, Burnside J, Melmed R, Civil R, Li Y. A randomized, double‐blind, multicenter, parallel‐group, placebo‐controlled, dose‐optimization study of the methylphenidate transdermal system for the treatment of ADHD in adolescents. CNS Spectrums 2010;15(7):419‐30. - PubMed
Fine 1993 {published data only}
-
- Fine S, Johnston C. Drug and placebo side effects in methylphenidate‐placebo trial for attention deficit hyperactivity disorder. Child Psychiatry and Human Development 1993;24(1):25‐30. - PubMed
-
- Johnston C, Fine S. Methods of evaluating methylphenidate in children with attention deficit hyperactivity disorder: acceptability, satisfaction and compliance. Journal of Pediatric Psychology 1993;18(6):717‐30. - PubMed
Firestone 1981 {published data only}
-
- Firestone P, Kelly MJ, Goodman JT, Davey J. Differential effects of parent training and stimulant medication with hyperactives: a progress report. Journal of the American Academy of Child and Adolescent Psychiatry 1981;20(1):135‐47. - PubMed
Fitzpatrick 1992a {published data only}
-
- Fitzpatrick PA, Klorman R, Brumaghim JT, Borgstedt AD. Effects of sustained‐release and standard preparations of methylphenidate on attention deficit disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31(2):226‐34. - PubMed
-
- Fitzpatrick PS. Effects of Sustained‐Release and Standard Preparations of Methylphenidate on Attention Deficit Hyperactivity Disorder: Clinical Outcome, Performance, and Cognitive Event‐Related Potentials [PhD thesis]. New York, USA: University of Rochester, 1990.
Flapper 2008 {published data only}
-
- Flapper BCT, Houwen S, Schoemaker MM. Fine motor skills and effects of methylphenidate in children with attention‐deficit‐hyperactivity disorder and developmental coordination disorder. Developmental Medicine & Child Neurology 2006;48(3):165‐9. - PubMed
-
- Flapper BCT, Schoemaker MM. Effects of methylphenidate on quality of life in children with both developmental coordination disorder and ADHD. Developmental Medicine & Child Neurology 2008;50(4):294‐9. - PubMed
Forness 1992 {published data only}
-
- Forness SR, Cantwell DP, Swanson JM, Hanna GL, Youpa D. Differential effects of stimulant medication on reading performance of boys with hyperactivity with and without conduct disorder. Journal of Learning Disabilities 1991;24(5):304‐10. - PubMed
-
- Forness SR, Swanson JM, Cantwell D, Guthrie D, Sena R. Response to stimulant medication across six measures of school‐related performance in children with ADHD and disruptive behavior. Behavioral Disorders 1992;18(1):42‐53.
Froehlich 2011 {published data only}
Gadow 1990 {published data only}
-
- Gadow KD, Nolan EE, Paolicelli LM, Sprafkin J. A procedure for assessing the effects of methylphenidate on hyperactive children in public school settings. Journal of Clinical Child Psychology 1991;20(3):268‐76.
-
- Gadow KD, Nolan EE, Sverd J, Sprafkin J, Paolicelli L. Methylphenidate in aggressive‐hyperactive boys: I. Effects on peer aggression in public school settings. Journal of the American Academy of Child and Adolescent Psychiatry 1990;29(5):710‐8. - PubMed
-
- Gadow KD, Paolicelli LM, Nolan EE, Schwartz J, Sprafkin J, Sverd J. Methylphenidate in aggressive hyperactive boys: II. Indirect effects of medication treatment on peer behavior. Journal of Child and Adolescent Psychopharmacology 1992;2(1):49‐61. - PubMed
Gadow 1995 {published data only}
-
- Gadow D, Nolan E, Sprafkin J, Sverd J. School observations of children with attention‐deficit hyperactivity disorder and comorbid tic disorder: effects of methylphenidate treatment. Journal of Developmental and Behavioral Pediatrics 1995;16(3):167–76. - PubMed
-
- Gadow D, Sverd J, Sprafkin J, Nolan E, Ezor N. Efficacy of methylphenidate for attention‐deficit hyperactivity disorder in children with tic disorder. Annual Progress in Child Psychiatry and Child Development. New York: Brunner/Mazel Publishers, 1996:494‐522.
-
- Gadow KD, Nolan EE, Sverd J. Methylphenidate in hyperactive boys with comorbid tic disorder: II. Short‐term behavioral effects in school settings. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31(3):462‐71. - PubMed
-
- Gadow KD, Nolan EE, Sverd J, Sprafkin J, Schwartz J. Anxiety and depression symptoms and response to methylphenidate in children with attention‐deficit hyperactivity disorder and tic disorder. Journal of Clinical Psychopharmacology 2002;22(3):267‐74. - PubMed
-
- Gadow KD, Sverd J, Sprafkin J, Nolan EE, Grossman S. Long‐term methylphenidate therapy in children with comorbid attention‐deficit hyperactivity disorder and chronic multiple tic disorder. Archives of General Psychiatry 1999;56(4):330‐6. - PubMed
Gadow 2007 {published data only}
-
- Gadow KD, Nolan E, Sprafkin J, Sverd J. School observations of children with attention‐deficit hyperactivity disorder and comorbid tic disorder: effects of methylphenidate treatment. Journal of Developmental and Behavioral Pediatrics 1995;16(3):167‐76. - PubMed
-
- Gadow KD, Nolan EE, Sverd J, Sprafkin J, Schneider J. Methylphenidate in children with oppositional defiant disorder and both comorbid chronic multiple tic disorder and ADHD. Journal of Child Neurology 2008;23(9):981‐90. - PubMed
-
- Gadow KD, Sverd J, Nolan EE, Sprafkin J, Schneider J. Immediate‐release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(7):840‐8. - PubMed
-
- Gadow KD, Sverd J, Sprafkin J, Nolan EE, Ezor SN. Efficacy of methylphenidate for attention‐deficit hyperactivity disorder in children with tic disorder. Archives of General Psychiatry 1995;52(6):444‐55. - PubMed
Gadow 2011 {published data only}
-
- Gadow KD, Nolan EE. Methylphenidate and comorbid anxiety disorder in children with both chronic multiple tic disorder and ADHD. Journal of Attention Disorders 2011;15(3):246‐56. - PubMed
Garfinkel 1983 {published data only}
-
- Garfinkel BD, Wender PH, Sloman L, O'Neill I. Tricyclic antidepressant and methylphenidate treatment of attention deficit disorder in children. Journal of the American Academy of Child and Adolescent Psychiatry 1983;22(4):343‐8. - PubMed
Gonzalez‐Heydrich 2010 {published data only}
-
- Gonzalez‐Heydrich J. OROS methylphenidate for attention‐deficit/hyperactivity disorder plus epilepsy. P and T 2006;31(12):725‐6. [EMBASE: 2007065321]
-
- Gonzalez‐Heydrich J, Whitney J, Hsin O, Mrakotsky C, MacMillan C, Torres A, et al. Tolerability of OROS‐MPH 18 and 36 mg in paediatric epilepsy plus attention deficit/hyperactivity disorder (ADHD). Epilepsia. Proceedings of the 26th International Epilepsy Congress; 2005 August 28th ‐ September 1st; Paris, France 2005;46(Suppl s6):179.
-
- NCT00323947. Methylphenidate for treating attention deficit hyperactivity disorder in children with both ADHD and epilepsy. www.clinicaltrials.gov (accessed 1 May 2013).
Gorman 2006 {published data only}
-
- Chang HTT. Effects of methylphenidate on performance and private speech of children with attention‐deficit/hyperactivity disorder during the Tower of Hanoi task. Dissertation Abstracts International: Section B: The Sciences and Engineering 2001;62(1‐B):540.
-
- Gorman EB, Klorman R, Thatcher JE, Borgstedt AD. Effects of methylphenidate on subtypes of attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(7):808‐16. - PubMed
-
- Kopecky H, Chang HT, Klorman R, Thatcher JE, Borgstedt AD. Performance and private speech of children with attention‐deficit/hyperactivity disorder while taking the Tower of Hanoi test: effects of depth of search, diagnostic subtype, and methylphenidate. Journal of Abnormal Child Psychology 2005;33(5):625‐38. - PubMed
Green 2011 {published data only}
-
- Green T, Weinberger R, Diamond A, Berant M, Hirschfeld L, Frisch A, et al. The effect of methylphenidate on prefrontal cognitive functioning, inattention, and hyperactivity in velocardiofacial syndrome. Journal of Child and Adolescent Psychopharmacology 2011;21(6):589‐95. - PubMed
-
- Green T, Weinberger R, Weizman A, Kotler M, Gothelf D. Effect of methylphenidate on neurocognitive functioning in velocardiofacial syndrome: a randomized placebo‐controlled trial. Biological Psychiatry 2009;65(Suppl 8):147.
Greenhill 2002 {published data only}
-
- Greenhill LL, Findling RL, Swanson JM, ADHD Study Group. A double‐blind, placebo‐controlled study of modified‐release methylphenidate in children with attention‐deficit/hyperactivity disorder. Pediatrics 2002;109(3):e39. - PubMed
Greenhill 2006 {published data only}
-
- Greenhill LL, Muniz R, Ball RR, Levine A, Pestreich L, Jiang H. Efficacy and safety of dexmethylphenidate extended‐release capsules in children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(7):817‐23. - PubMed
Grizenko 2012 {published data only}
-
- Fortier ME, Sengupta SM, Grizenko N, Choudhry Z, Thakur G, Joober R. Genetic evidence for the association of the hypothalamic‐pituitary‐adrenal (HPA) axis with ADHD and methylphenidate treatment response. Neuromolecular Medicine 2013;15(1):122‐32. - PubMed
-
- Grizenko N, Cai E, Jolicoeur C, Ter‐Stepanian M, Joober R. Effects of methylphenidate on acute math performance in children with attention‐deficit hyperactivity disorder. Canadian Journal of Psychiatry 2013;58(11):632‐9. - PubMed
-
- Grizenko N, Kovacina B, Amor LB, Schwartz G, Ter‐Stepanian M, Joober R, et al. Relationship between response to methylphenidate treatment in children with ADHD and psychopathology in their families. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(1):47‐53. - PubMed
Gruber 2007 {published data only}
-
- Gruber R, Grizenko N, Schwartz G, Benamor L, Ter‐Stepanian M, Joober R. The associations between sleep and attention in children with attention deficit hyperactivity disorder while on placebo and while on methylphenidate. Sleep. Proceedings of the 19th Annual Meeting of the Associated Professional Sleep Societies; 2005 June 18‐23; Denver, CO. Westchester: American Academy of Sleep Medicine, 2005; Vol. 28:A93‐4. [WOS: 000228906100277]
Hale 2011 {published data only}
-
- Hale JB, Reddy LA, Semrud‐Clikeman M, Hain LA, Whitaker J, Morley J, et al. Executive impairment determines ADHD medication response: implications for academic achievement. Journal of Learning Disabilities 2011;44(2):196‐212. - PubMed
-
- Kubas HA, Backenson EM, Wilcox G, Piercy JC, Hale JB. The effects of methylphenidate on cognitive function in children with attention‐deficit/hyperactivity disorder. Postgraduate Medicine 2012;124(5):33‐48. - PubMed
Heriot 2008 {published data only}
-
- Heriot SA, Evans IM, Foster TM. Critical influences affecting response to various treatments in young children with ADHD: a case series. Child: Care, Health and Development 2008;34(1):121‐33. - PubMed
Hicks 1985 {published data only}
-
- Gualtieri CT, Hicks RE, Mayo JP, Schroeder SR. The persistence of stimulant effects in chronically treated children: further evidence of an inverse relationship between drug effects and placebo levels of response. Psychopharmacology 1984;83(1):44‐7. - PubMed
-
- Hicks RE, Gualtieri CT, Mayo JP, Schroeder SR, Lipton MA. Methylphenidate and homeostasis: drug effects on the cognitive performance of hyperactive children. In: Bloomingdale Lewis M editor(s). Attention Deficit Disorder: Identification, Course and Treatment Rationale. New York, USA: SP Medical & Scientific Books, 1985:131‐41.
Hoeppner 1997 {published data only}
-
- Hoeppner JA, Hale JB, Bradley AM, Byrnes M, Coury DL, Lennie L, et al. A clinical protocol for determining methylphenidate dosage levels in ADHD. Journal of Attention Disorders 1997;2(1):19‐30.
Horn 1991 {published data only}
-
- Horn WF, Ialongo NS, Pascoe JM, Greenberg G, Packard T, Lopez M, et al. Additive effects of psychostimulants, parent training, and self‐control therapy with ADHD children. Journal of the American Academy of Child and Adolescent Psychiatry 1991;30(2):233‐40. - PubMed
-
- Ialongo NS, Horn WF, Pascoe JM, Greenberg G, Packard T, Lopez M, et al. The effects of a multimodal intervention with attention‐deficit hyperactivity disorder children: a 9‐month follow‐up. Journal of the American Academy of Child and Adolescent Psychiatry 1993;32(1):182‐9. - PubMed
Ialongo 1994 {published data only}
-
- Ialongo NS, Lopez M, Horn WF, Pascoe JM, Greenberg G. Effects of psychostimulant medication on self‐perceptions of competence, control, and mood in children with attention deficit hyperactivity disorder. Journal of Clinical Child Psychology 1994;23(2):161‐73.
Jacobi‐Polishook 2009 {published data only}
-
- Jacobi‐Polishook T, Shorer Z, Melzer I. The effect of methylphenidate on postural stability under single and dual task conditions in children with attention deficit hyperactivity disorder ‐ a double blind randomized control trial. Journal of the Neurological Sciences 2009;280(1‐2):15‐21. - PubMed
Jensen 1999 (MTA) {published data only}
-
- Abikoff H. Tailored psychosocial treatments for ADHD: the search for a good fit. Journal of Clinical Child Psychology 2001; Vol. 30, issue 1:122‐5. - PubMed
-
- Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott G, Greenhill LL, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Archives of General Psychiatry 1997;54(9):865‐70. - PubMed
-
- Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott GR, Greenhill LL, et al. NIMH Collaborative Multimodal Treatment Study of Children with ADHD (MTA): design, methodology, and protocol evolution. Journal of Attention Disorders 1997;2(3):141‐58.
-
- Arnold LE, Chuang S, Davies M, Abikoff HB, Conners CK, Elliott GR, et al. Nine months of multicomponent behavioral treatment for ADHD and effectiveness of MTA fading procedures. Journal of Abnormal Child Psychology 2004;32(1):39‐51. - PubMed
-
- Arnold LE, Elliott M, Sachs L, Bird H, Kraemer HC, Wells KC, et al. Effects of ethnicity on treatment attendance, stimulant response/dose, and 14‐month outcome in ADHD. Journal of Consulting and Clinical Psychology 2003;71(4):713‐27. - PubMed
Johnston 1988 {published data only}
-
- Johnston C, Pelham WE, Hoza J, Sturges J. Psychostimulant rebound in attention deficit disordered boys. Journal of the American Academy of Child and Adolescent Psychiatry 1988;27(6):806‐10. - PubMed
-
- Pelham WE Jr, Sturges J, Hoza J, Schmidt C, Bijlsma JJ, Milich R, et al. Sustained release and standard methylphenidate effects on cognitive and social behavior in children with attention deficit disorder. Pediatrics 1987;80(4):491‐501. - PubMed
-
- Pelham WE, Hoza J. Behavioral assessment of psychostimulant effects on ADD children in a summer day treatment program. In: Prinz R editor(s). Advances in Behavioral Assessment of Children and Families. Vol. 3, Greenwich, CT: JAI Press, 1987:3‐34.
-
- Pelham WE, Milich R, Walker JL. Effects of continuous and partial reinforcement and methylphenidate on learning in children with attention deficit disorder. Journal of Abnormal Psychology 1986;95(4):319‐25. - PubMed
Kaplan 1990 {published data only}
-
- Kaplan SL, Busner J, Kupietz S, Wassermann E, Segal B. Effects of methylphenidate on adolescents with aggressive conduct disorder and ADDH: a preliminary report. Journal of the American Academy of Child and Adolescent Psychiatry 1990;29(5):719‐23. - PubMed
Kelly 1989 {published data only}
-
- Kelly PC, Cohen ML, Walker WO, Caskey OL, Atkinson AW. Self‐esteem in children medically managed for attention deficit disorder. Pediatrics 1989;83(2):211‐7. - PubMed
Kent 1995 {published data only}
-
- Kent JD, Blader JC, Koplewicz HS, Abikoff H, Foley CA. Effects of late‐afternoon methylphenidate administration on behavior and sleep in attention‐deficit hyperactivity disorder. Pediatrics 1995;96(2):320‐5. - PubMed
Kent 1999 {published data only}
-
- Kent MA, Camfield CS, Camfield PR. Double‐blind methylphenidate trials: practical, useful, and highly endorsed by families. Archives of Pediatrics & Adolescent Medicine 1999;153(12):1292‐6. - PubMed
Klorman 1990 {published data only}
-
- Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD. Clinical effects of a controlled trial of methylphenidate on adolescents with attention deficit disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1990;29(5):702‐9. - PubMed
-
- Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD. Methylphenidate reduces abnormalities of stimulus classification in adolescents with attention deficit disorder. Journal of Abnormal Psychology 1992;101(1):130‐8. - PubMed
-
- Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD. Methylphenidate speeds evaluation processes of attention deficit disorder adolescents during a continuous performance test. Journal of Abnormal Child Psychology 1991;19(3):263‐83. - PubMed
Kolko 1999 {published data only}
-
- Kolko DJ, Bukstein OG, Barron J. Methylphenidate and behavior modification in children with ADHD and comorbid ODD or CD: main and incremental effects across settings. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(5):578‐86. - PubMed
Kollins 2006 (PATS) {published data only}
-
- At a glance... study design sought to balance rigor, subject safety. The Brown University Child & Adolescent Psychopharmacology Update 2006; Vol. 8, issue 12:4‐5.
-
- MPH‐related reductions in growth rates. The Brown University Child & Adolescent Psychopharmacology Update 2006; Vol. 8, issue 12:6.
-
- PATS shows mixed effect of medication on functional outcomes. The Brown University Child & Adolescent Psychopharmacology Update 2008; Vol. 10, issue 2:6.
-
- PATS: efficacy of MPH in ADHD preschoolers. The Brown University Child & Adolescent Psychopharmacology Update 2006; Vol. 8, issue 12:5‐6.
-
- PATS: safety and tolerability of MPH in ADHD preschoolers. The Brown University Child & Adolescent Psychopharmacology Update 2006; Vol. 8, issue 12:2‐4.
Konrad 2004 {published data only}
-
- Konrad K, Günther T, Hanisch C, Herpertz‐Dahlmann B. Differential effects of methylphenidate on attentional functions in children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(2):191‐8. - PubMed
Konrad 2005 {published data only}
-
- Konrad K, Günther T, Heinzel‐Gutenbrunner M, Herpertz‐Dahlmann B. Clinical evaluation of subjective and objective changes in motor activity and attention in children with attention‐deficit/hyperactivity disorder in a double‐blind methylphenidate trial. Journal of Child and Adolescent Psychopharmacology 2005;15(2):180‐90. - PubMed
Leddy 2009 {published data only}
-
- Leddy JJ, Waxmonsky JG, Salis RJ, Paluch RA, Gnagy EM, Mahaney P, et al. Dopamine‐related genotypes and the dose‐response effect of methylphenidate on eating in attention‐deficit/hyperactivity disorder youths. Journal of Child and Adolescent Psychopharmacology 2009;19(2):127‐36. - PubMed
Lehmkuhl 2002 {published data only}
-
- Döpfner M, Banaschewski T, Schmidt J, Uebel H, Schmeck K, Gerber WD, et al. Long‐acting methylphenidate preparation in children with ADHD ‐ a multicenter study [Langzeitwirksames methylphenidat bei kindern mit aufmerksamkeitsdefizit‐hyperaktivitätsstörungen: eine multizentrische studie]. Nervenheilkunde: Zeitschrift fur interdisziplinaere Fortbildung 2003;22(2):85‐92.
-
- Lehmkuhl G. Placebo‐controlled, double‐blind multicenter trial on the efficacy of sustained‐release methylphenidate in children suffering from attention deficit hyperactivity disorder (ADHD), Phase III. Integrated Final Report. Institut für medizinische Informatik, Biometrie und Epidemiologie, Universitätsklinikum Essen, Trial no 6520‐9979‐02, 1‐9 2002.
-
- Sinzig J, Döpfner M, Lehmkuhl G, Uebel H, Schmeck K, Poustka F, et al. Long‐acting methylphenidate has an effect on aggressive behavior in children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2007;17(4):421‐32. - PubMed
-
- Sinzig JK, Döpfner M, Plück J, Banaschewski T, Stephani U, Lehmkuhl G, et al. Does a morning dose of methylphenidate retard reduce hyperkinetic symptoms in the afternoon? [Lassen sich hyperkinetische auffälligkeiten am nachmittag durch eine morgengabe von methylphenidat retard vermindern?]. Zeitschrift für Kinder‐ und Jugendpsychiatrie und Psychotherapie 2004;32(4):225‐33. - PubMed
Lijffijt 2006 {published data only}
-
- Lijffijt M, Kenemans JL, Ter Wal A, Quik EH, Kemner C, Westenberg H, et al. Dose‐related effect of methylphenidate on stopping and changing in children with attention‐deficit/hyperactivity disorder. European Psychiatry 2006;21(8):544‐7. - PubMed
Lin 2014 {published data only}
Lopez 2003 {published data only}
-
- Lopez F, Silva R, Pestreich L, Muniz R. Comparative efficacy of two once daily methylphenidate formulations (Ritalin® LA™1 and Concerta®) and placebo in children with attention deficit hyperactivity disorder across the school day. Paediatric Drugs 2003;5(8):545‐55. - PubMed
-
- Lopez F, Silva R, Pestreich L, Muniz R. Erratum for: Comparative efficacy of two once daily methylphenidate formulations (Ritalin LA1 and Concerta) and placebo in children with attention deficit hyperactivity disorder across the school day. Pediatric Drugs 2003;5(12):832. Erratum for Pediatric Drugs 2003;5:545. [EMBASE: 2004010793] - PubMed
-
- Lopez FA, Silva RR, Pestreich L, Lee J, Muniz R. Comparative school‐day efficacy of Ritalin LA, Concerta, and placebo in children with attention deficit hyperactivity disorder. Annals of Neurology. Proceedings of the 32nd Annual Meeting of the Child Neurology Society; 2003 October 1‐4; Miami Beach, Florida. New York: John Wiley & Sons, 2003; Vol. 54 (Suppl 7):S143. [WOS: 000185260300393]
Lufi 1997 {published data only}
-
- Lufi D, Parish‐Plass J, Gai E. The effect of methylphenidate on the cognitive and personality functioning of ADHD children. Israel Journal of Psychiatry and Related Sciences 1997;34(3):200‐9. - PubMed
Lufi 2007 {published data only}
-
- Lufi D, Gai E. The effect of methylphenidate and placebo on eye‐hand coordination functioning and handwriting of children with attention deficit hyperactivity disorder. Neurocase 2007;13(5):334‐41. - PubMed
Manos 1999 {published data only}
-
- Faraone SV, Short EJ, Biederman J, Findling RL, Roe C, Manos MJ. Efficacy of Adderall and methylphenidate in attention deficit hyperactivity disorder: a drug‐placebo and drug‐drug response curve analysis of a naturalistic study. International Journal of Neuropsychopharmacology 2002;5(2):121‐9. - PubMed
-
- Findling RL, Short EJ, Manos MJ. Developmental aspects of psychostimulant treatment in children and adolescents with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(12):1441‐7. - PubMed
-
- Findling RL, Short EJ, Manos MJ. Short‐term cardiovascular effects of methylphenidate and Adderall. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(5):525‐9. - PubMed
-
- Manos MJ, Short EJ, Findling RL. Differential effectiveness of methylphenidate and Adderall in school‐age youths with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(7):813‐9. - PubMed
Martins 2004 {published data only}
-
- Martins S, Tramontina S, Polanczyk G, Eizirik M, Swanson JM, Rohde LA. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. Journal of Child and Adolescent Psychopharmacology 2004;14(2):195‐206. - PubMed
McBride 1988a {published data only}
-
- McBride MC. An individual double‐blind crossover trial for assessing methylphenidate response in children with attention deficit disorder. Journal of Pediatrics 1988;113(1 Pt 1):137‐45. - PubMed
McGough 2006 {published data only}
-
- McGough JJ, Wigal SB, Abikoff H, Turnbow JM, Posner K, Moon E. A randomized, double‐blind, placebo‐controlled, laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. Journal of Attention Disorders 2006;9(3):476‐85. - PubMed
-
- Wigal S, Turnbow J, Abikoff H, McGough J, Cohen J. Parent rated effects of transdermal methylphenidate in children with ADHD. International Journal of Neuropsychopharmacology. Abstracts from the XXVI Collegium Internationale Neuro‐Psychopharmacologicum; 2008 July 13‐17; Munich. 2008; Vol. 11 Suppl 1:232.
McInnes 2007 {published data only}
-
- McInnes A, Bedard A‐C, Hogg‐Johnson S, Tannock R. Preliminary evidence of beneficial effects of methylphenidate on listening comprehension in children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2007;17(1):35‐49. - PubMed
Moshe 2012 {published data only}
-
- Moshe K, Karni A, Tirosh E. Anxiety and methylphenidate in attention deficit hyperactivity disorder: a double‐blind placebo‐drug trial. Attention Deficit and Hyperactivity Disorders 2012;4(3):153‐8. - PubMed
Muniz 2008 {published data only}
-
- Muniz R, Brams M, Mao A, McCague K, Pestreich L, Silva R. Efficacy and safety of extended‐release dexmethylphenidate compared with d,l‐methylphenidate and placebo in the treatment of children with attention‐deficit/hyperactivity disorder: a 12‐hour laboratory classroom study. Journal of Child and Adolescent Psychopharmacology 2008;18(3):248‐56. - PubMed
-
- Silva R, Muniz R, McCague K, Childress A, Brams M, Mao A. Treatment of children with attention‐deficit/hyperactivity disorder: results of a randomized, multicenter, double‐blind, crossover study of extended‐release dexmethylphenidate and D,L‐methylphenidate and placebo in a laboratory classroom setting. Psychopharmacology Bulletin 2008;41(1):19‐33. - PubMed
Murray 2011 {published data only}
-
- Murray DW, Childress A, Giblin J, Williamson D, Armstrong R, Starr HL. Effects of OROS methylphenidate on academic, behavioral, and cognitive tasks in children 9 to 12 years of age with attention‐deficit/hyperactivity disorder. Clinical Pediatrics 2011;50(4):308‐20. - PubMed
Musten 1997 {published data only}
-
- Firestone P, Musten LM, Pisterman S, Mercer J, Bennett S. Short‐term side effects of stimulant medication are increased in preschool children with attention‐deficit/hyperactivity disorder: a double‐blind placebo‐controlled study. Journal of Child and Adolescent Psychopharmacology 1998;8(1):13‐25. - PubMed
-
- Musten LM. Efficacy of stimulant medication treatment of attention deficit hyperactivity disorder in preschool‐aged children. Dissertation Abstracts International: Section B: The Sciences and Engineering 1998; Vol. 59, issue 3‐B:1374.
-
- Musten LM, Firestone P, Pisterman S, Bennett S, Mercer J. Effects of methylphenidate on preschool children with ADHD: cognitive and behavioral functions. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(10):1407‐15. - PubMed
Newcorn 2008 {published data only}
-
- Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. American Journal of Psychiatry 2008;165(6):721‐30. - PubMed
-
- Newcorn JH, Kratochvil CJ, Allen AJ, et al. Osmotically released methylphenidate compared to atomoxetine for ADHD. The Brown University Child & Adolescent Psychopharmacology Update 2008; Vol. 10, issue 8:1.
-
- Toplak ME. Osmotically released methylphenidate is more effective than atomoxetine in children and adolescents with ADHD. Evidence‐Based Mental Health 2009;12(1):19. - PubMed
Nikles 2006 {published data only}
-
- Nikles CJ, Mitchell GK, Mar CB, Clavarino A, McNairn N. An n‐of‐1 trial service in clinical practice: testing the effectiveness of stimulants for attention‐deficit/hyperactivity disorder. Pediatrics 2006;117(6):2040‐6. - PubMed
-
- Nikles CJ, Mitchell GK, Mar CB, McNairn N, Clavarino A. Long‐term changes in management following n‐of‐1 trials of stimulants in attention‐deficit/hyperactivity disorder. European Journal of Clinical Pharmacology 2007;63(11):985‐9. - PubMed
Oesterheld 1998 {published data only}
-
- Oesterheld JR, Kofoed L, Tervo R, Fogas B, Wilson A, Fiechtner H. Effectiveness of methylphenidate in Native American children with fetal alcohol syndrome and attention deficit/hyperactivity disorder: a controlled pilot study. Journal of Child and Adolescent Psychopharmacology 1998;8(1):39‐48. - PubMed
Overtoom 2003 {published data only}
-
- Overtoom CCE, Verbaten MN, Kemner C, Kenemans JL, Engeland H, Buitelaar JK, et al. Effects of methylphenidate, desipramine, and L‐dopa on attention and inhibition in children with attention deficit hyperactivity disorder. Behavioural Brain Research 2003;145(1‐2):7‐15. - PubMed
Palumbo 2008 {published data only}
-
- Daviss WB, Patel NC, Robb AS, McDermott MP, Bukstein OG, Pelham WE, et al. Clonidine for attention‐deficit/hyperactivity disorder: II. ECG changes and adverse events analysis. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(2):189‐98. - PubMed
-
- Palumbo DR, Sallee FR, Pelham WE, Bukstein OG, Daviss WB, McDermott MP. Clonidine for attention‐deficit/hyperactivity disorder: I. Efficacy and tolerability outcomes. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(2):180‐8. - PubMed
Pearson 2013 {published data only}
-
- Methylphenidate dosing improved behavior in children with ASD. The Brown University Child & Adolescent Psychopharmacology Update 2013; Vol. 15, issue 8:4‐5.
-
- Pearson DA, Santos CW, Aman MG, Arnold LE, Casat CD, Mansour R, et al. Effects of extended release methylphenidate treatment on ratings of attention‐deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. Journal of Child and Adolescent Psychopharmacology 2013;23(5):337‐51. - PMC - PubMed
Pelham 1989 {published data only}
-
- Pelham WE, Walker JL, Sturges J, Hoza J. Comparative effects of methylphenidate on ADD girls and ADD boys. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(5):773‐6. - PubMed
Pelham 1990a {published data only}
-
- Pelham Jr WE, Greenslade KE, Vodde‐Hamilton M, Murphy DA, Greenstein JJ, Gnagy EM, et al. Relative efficacy of long‐acting stimulants on children with attention deficit‐hyperactivity disorder: a comparison of standard methylphenidate, sustained‐release methylphenidate, sustained‐release dextroamphetamine, and pemoline. Pediatrics 1990;86(2):226‐37. - PubMed
Pelham 1993a {published data only}
-
- Pelham J, Carlson C, Sams SE, Vallano G, Dixon MJ, Hoza B, et al. Separate and combined effects of methylphenidate and behavior modification on boys with attention deficit‐hyperactivity disorder in the classroom. Journal of Consulting and Clinical Psychology 1993;61(3):506‐15. - PubMed
Pelham 1999 {published data only}
-
- Pelham WE, Aronoff HR, Midlam JK, Shapiro CJ, Gnagy EM, Chronis AM, et al. A comparison of Ritalin and Adderall: efficacy and time‐course in children with attention‐deficit/hyperactivity disorder. Pediatrics 1999;103(4):e43. - PubMed
Pelham 2001a {published data only}
-
- Pelham WE, Gnagy EM, Burrows‐Maclean L, Williams A, Fabiano GA, Morrisey SM, et al. Once‐a‐day Concerta methylphenidate versus three‐times‐daily methylphenidate in laboratory and natural settings. Pediatrics 2001;107(6):E105. - PubMed
Pelham 2002 {published data only}
-
- Pelham WE, Hoza B, Pillow DR, Gnagy EM, Kipp HL, Greiner AR, et al. Effects of methylphenidate and expectancy on children with ADHD: behavior, academic performance, and attributions in a summer treatment program and regular classroom settings. Journal of Consulting and Clinical Psychology 2002;70(2):320‐35. - PubMed
Pelham 2005 {published data only}
-
- Chacko A, Pelham WE, Gnagy EM, Greiner A, Vallano G, Bukstein O, et al. Stimulant medication effects in a summer treatment program among young children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2005;44(3):249‐57. - PubMed
-
- Pelham WE, Burrows‐MacLean L, Gnagy EM, Fabiano GA, Coles EK, Tresco KE, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Experimental and Clinical Psychopharmacology 2005;13(2):111‐26. - PubMed
-
- Pelham WE, Manos MJ, Ezzell CE, Tresco KE, Gnagy EM, Hoffman MT, et al. A dose‐ranging study of a methylphenidate transdermal system in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2005;44(6):522‐9. - PubMed
Pelham 2011 {published data only}
-
- Pelham WE, Waxmonsky JG, Schentag J, Ballow CH, Panahon CJ, Gnagy EM, et al. Efficacy of a methylphenidate transdermal system versus t.i.d. methylphenidate in a laboratory setting. Journal of Attention Disorders 2011;15(1):28‐35. - PubMed
Pelham 2014 {published data only}
Perez‐Alvarez 2009 {published data only}
-
- Perez‐Alvarez F, Serra‐Amaya C, Timoneda‐Gallart CA. Cognitive versus behavioral ADHD phenotype: what is it all about?. Neuropediatrics 2009;40(1):32‐8. - PubMed
Pliszka 1990 {published data only}
-
- Pliszka SR. Effect of anxiety on cognition, behavior, and stimulant response in ADHD. In: Chess S, Hertzig ME editor(s). Annual Progress in Child Psychiatry and Child Development. London: Routledge, 1990:454‐66. - PubMed
-
- Pliszka SR. Effect of anxiety on cognition, behavior, and stimulant response in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(6):882‐7. - PubMed
Pliszka 2000 {published data only}
-
- Pliszka SR, Browne RG, Olvera RL, Wynne SK. A double‐blind, placebo‐controlled study of Adderall and methylphenidate in the treatment of attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39(5):619‐26. - PubMed
Pliszka 2007 {published data only}
-
- Pliszka SR, Liotti M, Bailey BY, Perez R, Glahn D, Semrud‐Clikeman M. Electrophysiological effects of stimulant treatment on inhibitory control in children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2007;17(3):356‐66. - PubMed
Quinn 2004 {published data only}
-
- Quinn D, Wigal S, Swanson J, Hirsch S, Ottolini Y, Dariani M, et al. Comparative pharmacodynamics and plasma concentrations of d‐threo‐methylphenidate hydrochloride after single doses of d‐threo‐methylphenidate hydrochloride and d,l‐threo‐methylphenidate hydrochloride in a double‐blind, placebo‐controlled, crossover laboratory school study in children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(11):1422‐9. - PubMed
Ramtvedt 2013 {published data only}
-
- Ramtvedt BE, Sandvik L, Sundet K. Correspondence between children's and adults' ratings of stimulant‐induced changes in ADHD behaviours in a crossover trial with medication‐naive children. European Journal of Developmental Psychology 2014;11(6):687‐700.
-
- Ramtvedt BE, Sundet K. Relationships between computer‐based testing and behavioral ratings in the assessment of attention and activity in a pediatric ADHD stimulant crossover trial. Clinical Neuropsychologist 2014;28(7):1146‐61. - PubMed
Rapport 1985 {published data only}
-
- Rapport MD, DuPaul GJ. Methylphenidate: rate‐dependent effects on hyperactivity. Psychopharmacology Bulletin 1986;22(1):223‐8. - PubMed
-
- Rapport MD, DuPaul GJ, Stoner G, Birmingham BK, Masse G. Attention deficit disorder with hyperactivity: differential effects of methylphenidate on impulsivity. Pediatrics 1985;76(6):938‐43. - PubMed
-
- Rapport MD, DuPaul GJ, Stoner G, Jones TJ. Comparing classroom and clinic measures of attention deficit disorder: differential, idiosyncratic, and dose‐response effects of methylphenidate. Journal of Consulting and Clinical Psychology 1986;54(3):334‐41. - PubMed
-
- Rapport MD, Stoner G, DuPaul GJ, Birmingham BK, Tucker S. Methylphenidate in hyperactive children: differential effects of dose on academic, learning, and social behavior. Journal of Abnormal Child Psychology 1985;13(2):227‐43. - PubMed
Rapport 1987 {published data only}
-
- Denney CB, Rapport MD. Predicting methylphenidate response in children with ADHD: theoretical, empirical, and conceptual models. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(4):393‐401. - PubMed
-
- DuPaul GJ, Rapport MD. Does methylphenidate normalize the classroom performance of children with attention deficit disorder?. Journal of the American Academy of Child and Adolescent Psychiatry 1993;32(1):190‐8. - PubMed
-
- DuPaul GJ, Rapport MD, Vyse SA. ADHD and methylphenidate responders: effects on behavior controlled by complex reinforcement schedules. International Clinical Psychopharmacology 1988;3(4):349‐61. - PubMed
-
- Kelly KL, Rapport MD, DuPaul GJ. Attention deficit disorder and methylphenidate: a multi‐step analysis of dose‐response effects on children's cardiovascular functioning. International Clinical Psychopharmacology 1988;3(2):167‐81. - PubMed
-
- Rapport MD, Denney C. Titrating methylphenidate in children with attention‐deficit/hyperactivity disorder: is body mass predictive of clinical response?. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(4):523‐30. - PubMed
Rapport 2008 {published data only}
-
- Rapport MD, Kofler MJ, Coiro MM, Raiker JS, Sarver DE, Alderson RM. Unexpected effects of methylphenidate in attention‐deficit/hyperactivity disorder reflect decreases in core/secondary symptoms and physical complaints common to all children. Journal of Child and Adolescent Psychopharmacology 2008;18(3):237‐47. - PubMed
Riggs 2011 {published data only}
-
- Riggs PD, Winhusen T, Davies RD, Leimberger JD, Mikulich‐Gilbertson S, Klein C, et al. Randomized controlled trial of osmotic‐release methylphenidate with cognitive‐behavioral therapy in adolescents with attention‐deficit/hyperactivity disorder and substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2011;50(9):903‐14. - PMC - PubMed
-
- Winhusen TM, Lewis DF, Riggs PD, Davies RD, Adler LA, Sonne S, et al. Subjective effects, misuse, and adverse effects of osmotic‐release methylphenidate treatment in adolescent substance abusers with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2011;21(5):455‐63. - PMC - PubMed
Rubinsten 2008 {published data only}
-
- Rubinsten O, Bedard A‐C, Tannock R. Methylphenidate has differential effects on numerical abilities in ADHD children with and without co‐morbid mathematical difficulties. Open Psychology Journal 2008;1:11‐7.
Samuels 2006 {published data only}
-
- Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24‐h ambulatory blood pressure in children with ADHD: a double‐blind, randomized, cross‐over trial. Pediatric Nephrology 2006;21(1):92‐5. - PubMed
Schachar 1997a {published data only}
-
- Charach A, Figueroa M, Chen S, Ickowicz A, Schachar R. Stimulant treatment over 5 years: effects on growth. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(4):415‐21. - PubMed
-
- Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(5):559‐67. - PubMed
-
- Diamond IR, Tannock R, Schachar RJ. Response to methylphenidate in children with ADHD and comorbid anxiety. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(4):402‐9. - PubMed
-
- Law SF, Schachar RJ. Do typical clinical doses of methylphenidate cause tics in children treated for attention‐deficit hyperactivity disorder?. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(8):944‐51. - PubMed
-
- Schachar RJ, Tannock R, Cunningham C, Corkum PV. Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(6):754‐63. - PubMed
Schachar 2008 {published data only}
-
- Schachar R, Ickowicz A, Crosbie J, Donnelly GAE, Reiz JL, Miceli PC, et al. Cognitive and behavioral effects of multilayer‐release methylphenidate in the treatment of children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2008;18(1):11‐24. - PubMed
Schulz 2010 {published data only}
-
- Schulz E, Fleischhaker C, Hennighausen K, Heiser P, Oehler K‐U, Linder M, et al. A double‐blind, randomized, placebo/active controlled crossover evaluation of the efficacy and safety of Ritalin® LA in children with attention‐deficit/hyperactivity disorder in a laboratory classroom setting. Journal of Child and Adolescent Psychopharmacology 2010;20(5):377‐85. - PubMed
Schwartz 2004 {published data only}
-
- Gruber R, Grizenko N, Schwartz G, Ben Amor L, Gauthier J, Guzman R, et al. Sleep and COMT polymorphism in ADHD children: preliminary actigraphic data. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(8):982‐9. - PubMed
-
- Schwartz G, Amor LB, Grizenko N, Lageix P, Baron C, Boivin DB, et al. Actigraphic monitoring during sleep of children with ADHD on methylphenidate and placebo. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(10):1276‐82. - PubMed
Sharp 1999 {published data only}
-
- Borcherding BG, Keysor CS, Cooper TB, Rapoport JL. Differential effects of methylphenidate and dextroamphetamine on the motor activity level of hyperactive children. Neuropsychopharmacology 1989;2(4):255‐63. - PubMed
-
- Castellanos FX, Elia J, Kruesi MJ, Marsh WL, Gulotta CS, Potter WZ, et al. Cerebrospinal fluid homovanillic acid predicts behavioral response to stimulants in 45 boys with attention deficit/hyperactivity disorder. Neuropsychopharmacology 1996;14(2):125‐37. - PubMed
-
- Elia J, Borcherding BG, Rapoport JL, Keysor CS. Methylphenidate and dextroamphetamine treatments of hyperactivity: are there true nonresponders?. Psychiatry Research 1991;36(2):141‐55. - PubMed
-
- Elia J, Welsh PA, Gullotta CS, Rapoport JL. Classroom academic performance: improvement with both methylphenidate and dextroamphetamine in ADHD boys. Journal of Child Psychology and Psychiatry, and Allied Disciplines 1993;34(5):785‐804. - PubMed
-
- Schmidt ME, Kruesi MJ, Elia J, Borcherding BG, Elin RJ, Hosseini JM, et al. Effect of dextroamphetamine and methylphenidate on calcium and magnesium concentration in hyperactive boys. Psychiatry Research 1994;54(2):199‐210. - PubMed
Shiels 2009 {published data only}
Silva 2005a {published data only}
-
- Silva R, Muniz R, Pestreich LK, Brams M, Childress A, Lopez FA. Efficacy of two long‐acting methylphenidate formulations in children with attention‐deficit/hyperactivity disorder in a laboratory classroom setting. Journal of Child and Adolescent Psychopharmacology 2005;15(4):637‐54. - PubMed
Silva 2006 {published data only}
-
- Silva RR, Muniz R, Pestreich L, Childress A, Brams M, Lopez FA, et al. Efficacy and duration of effect of extended‐release dexmethylphenidate versus placebo in schoolchildren with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2006;16(3):239‐51. - PubMed
Silva 2008 {published data only}
-
- Silva RR, Muniz R, Pestreich L, Brams M, Mao AR, Childress A, et al. Dexmethylphenidate extended‐release capsules in children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(2):199‐208. - PubMed
-
- Silva RR, Muniz R, Pestreich L, Lopez FA, Childress A, Wang J. Once‐daily dexmethylphenidate: a placebo‐controlled crossover study in children with attention‐deficit/hyperactivity disorder. Annals of Neurology 2005;58(Suppl S9):S109.
Smith 1998 {published data only}
-
- Evans SW, Pelham WE, Smith BH, Bukstein O, Gnagy EM, Greiner AR, et al. Dose‐response effects of methylphenidate on ecologically valid measures of academic performance and classroom behavior in adolescents with ADHD. Experimental and Clinical Psychopharmacology 2001;9(2):163‐75. - PubMed
-
- Smith BH. Reliability, validity and unique contributions of self‐reports by adolescents being treated for attention‐deficit hyperactivity disorder. Dissertation Abstracts International: Section B: The Sciences and Engineering. US: U Arizona, 1997; Vol. 58, issue 6‐B:3328.
-
- Smith BH, Pelham WE, Evans S, Gnagy E, Molina B, Bukstein O, et al. Dosage effects of methylphenidate on the social behavior of adolescents diagnosed with attention‐deficit hyperactivity disorder. Experimental and Clinical Psychopharmacology 1998;6(2):187‐204. - PubMed
-
- Smith BH, Pelham WE, Gnagy E, Yudell RS. Equivalent effects of stimulant treatment for attention‐deficit hyperactivity disorder during childhood and adolescence. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37(3):314‐21. - PubMed
Smith 2004 {published data only}
-
- Smith R, Larsen D, Derby KM, McLaughlin TF, Weber KP, Brown K, et al. A comparison of teacher checklists used over 15 days and a one‐day antecedent analysis to conduct a medication trial. Psychology in the Schools 2004;41(2):235‐40.
Smithee 1998 {published data only}
-
- Smithee JA, Klorman R, Brumaghim JT, Borgstedt AD. Methylphenidate does not modify the impact of response frequency or stimulus sequence on performance and event‐related potentials of children with attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology 1998;26(4):233‐45. - PubMed
Solanto 2009 {published data only}
Stein 1996 {published data only}
-
- Stein MA, Blondis TA, Schnitzler ER, O'Brien T, Fishkin J, Blackwell B, et al. Methylphenidate dosing: twice daily versus three times daily. Pediatrics 1996;98(4 Pt 1):748‐56. - PubMed
Stein 2003 {published data only}
-
- Stein MA, Sarampote C, Seymour K. Insomnia and tiredness in ADHD youth: relationship with methylphenidate dose, age, and weight. Pediatric Research. Annual Meeting of the Pediatric Academic Societies; 2004 May 4; San Francisco, CA. Baltimore: International Pediatric Research Foundation, 2004; Vol. 55 (4):74A. [WOS: 000220591100437]
-
- Stein MA, Sarampote CS, Waldman ID, Robb AS, Conlon C, Pearl PL, et al. A dose‐response study of OROS methylphenidate in children with attention‐deficit/hyperactivity disorder. Pediatrics 2003;112(5):e404. - PubMed
-
- Stein MA, Seymour KE, Black DO, Sarampote CS, Robb A, Conlon C, et al. Effects and side effects of Concerta methylphenidate (MPH) in children with ADHD and comorbid internalizing symptoms. Pediatric Research. Annual Meeting of the Pediatric Academic Society; 2003 May 3‐6; Seattle, Washington. Baltimore: International Pediatric Research Foundation, 2003; Vol. 53 (4):555A. [WOS: 000181897903139]
-
- Stein MA, Waldman ID, Sarampote CS, Seymour KE, Robb AS, Conlon C, et al. Dopamine transporter genotype and methylphenidate dose response in children with ADHD. Neuropsychopharmacology 2005;30(7):1374‐82. - PubMed
Stein 2011 {published data only}
-
- Wiebe S, Gruber R, Charney E, Aryal S, Waldman I, Newcorn J, et al. Sleep and emotional reactivity to extended release dexmethylphenidate versus mixed amphetamine salts: a double‐blind, placebo controlled study. European Child & Adolescent Psychiatry. Proceedings of the Eunethydis 1st International ADHD Conference: From Data to Best Clinical Practice; 2010 May 26‐28; Amsterdam, Netherlands 2010;19(Suppl 1):S82.
Stoner 1994 {published data only}
Sumner 2010 {published data only}
-
- Sumner CR, Haynes VS, Teicher MH, Newcorn JH. Does placebo response differ between objective and subjective measures in children with attention‐deficit/hyperactivity disorder?. Postgraduate Medicine 2010;122(5):52‐61. - PubMed
Sunohara 1999 {published data only}
-
- Sunohara GA. Methylphenidate effects on focused and selective attention processing in children with ADHD. Dissertation Abstracts International: Section B: The Sciences and Engineering 1998; Vol. 59, issue 6‐B:3113.
-
- Sunohara GA, Malone MA, Rovet J, Humphries T, Roberts W, Taylor MJ. Effect of methylphenidate on attention in children with attention deficit hyperactivity disorder (ADHD): ERP evidence. Neuropsychopharmacology 1999;21(2):218‐28. - PubMed
Swanson 1998 {published data only}
-
- Swanson J, Wigal S, Greenhill L, Browne R, Waslick B, Lerner M, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacology Bulletin 1998;34(1):55‐60. - PubMed
-
- Swanson JM, Wigal S, Greenhill LL, Browne R, Waslik B, Lerner M, et al. Analog classroom assessment of Adderall® in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37(5):519‐26. - PubMed
Swanson 1999 {published data only}
-
- Swanson J, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, et al. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clinical Pharmacology and Therapeutics 1999;66(3):295‐305. - PubMed
-
- Wigal SB, Gupta S, Guinta D, Swanson JM. Reliability and validity of the SKAMP rating scale in a laboratory school setting. Psychopharmacology Bulletin 1998;34(1):47‐53. - PubMed
Swanson 2002a {published data only}
-
- Swanson J, Gupta S, Lam A, Shoulson I, Lerner M, Modi N, et al. Development of a new once‐a‐day formulation of methylphenidate for the treatment of attention‐deficit/hyperactivity disorder: proof‐of‐concept and proof‐of‐product studies. Archives of General Psychiatry 2003;60(2):204‐11. - PubMed
-
- Swanson J, Sadeh A, Lerner MA, Wigal SB. Comparison of the impact of OROS methylphenidate HCI with methylphenidate tid and placebo on the sleep of children with ADHD. Journal of Developmental and Behavioral Pediatrics 2000;21(5):387‐8.
-
- Swanson JM, Gupta S, Williams L, Agler D, Lerner M, Wigal S. Efficacy of a new pattern of delivery of methylphenidate for the treatment of ADHD: effects on activity level in the classroom and on the playground. Journal of the American Academy of Child and Adolescent Psychiatry 2002;41(11):1306‐14. - PubMed
-
- Swanson JM, Wigal SB, Lemer MA. Comparison of the efficacy and safety of OROS methylphenidate HCI with methylphenidate tid and placebo in children with ADHD. Pediatric Research 2000;47(4):34A. [WOS: 000086155300200]
-
- Wigal S, Lerner M, Swanson J. Once‐daily methylphenidate formulation: impact on academic productivity and activity levels of children with attention‐deficit/hyperactivity disorder. European Neuropsychopharmacology 2002;12(Suppl 3):416.
Swanson 2002b {published data only}
-
- Swanson J, Gupta S, Lam A, Shoulson I, Lerner M, Modi N, et al. Development of a new once‐a‐day formulation of methylphenidate for the treatment of attention‐deficit/hyperactivity disorder: proof‐of‐concept and proof‐of‐product studies. Archives of General Psychiatry 2003;60(2):204‐11. - PubMed
-
- Swanson J, Sadeh A, Lerner MA, Wigal SB. Comparison of the impact of OROS methylphenidate HCI with methylphenidate tid and placebo on the sleep of children with ADHD. Journal of Developmental and Behavioral Pediatrics 2000;21(5):387‐8. [WOS: 000089857900028]
-
- Swanson J, Wigal S, Lerner M. Treatment with a controlled‐release formulation of methylphenidate for attention‐deficit/hyperactivity disorder: onset and duration of effect. European Neuropsychopharmacology 2002;12(Suppl 3):414.
-
- Swanson JM, Gupta S, Williams L, Agler D, Lerner M, Wigal S. Efficacy of a new pattern of delivery of methylphenidate for the treatment of ADHD: effects on activity level in the classroom and on the playground. Journal of the American Academy of Child and Adolescent Psychiatry 2002;41(11):1306‐14. - PubMed
-
- Swanson JM, Wigal SB, Lemer MA. Comparison of the efficacy and safety of OROS methylphenidate HCI with methylphenidate tid and placebo in children with ADHD. Pediatric Research 2000;47(Suppl):34A.
Swanson 2004b {published data only}
-
- Sonuga‐Barke EJ, Coghill D, Markowitz JS, Swanson JM, Vandenberghe M, Hatch SJ. Sex differences in the response of children with ADHD to once‐daily formulations of methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(6):701‐10. - PubMed
-
- Sonuga‐Barke EJ, Lier P, Swanson JM, Coghill D, Wigal S, Vandenberghe M, et al. Heterogeneity in the pharmacodynamics of two long‐acting methylphenidate formulations for children with attention deficit/hyperactivity disorder. A growth mixture modelling analysis. European Child & Adolescent Psychiatry 2008;17(4):245‐54. - PubMed
-
- Sonuga‐Barke EJS, Coghill D, Wigal T, DeBacker M, Swanson J. Adverse reactions to methylphenidate treatment for attention‐deficit/hyperactivity disorder: structure and associations with clinical characteristics and symptom control. Journal of Child and Adolescent Psychopharmacology 2009;19(6):683‐90. - PubMed
-
- Sonuge‐Barke EJS, Swanson J, Hatch S, Lier P, Vandenberghe M. Heterogeneity in ADHD children's response to two long‐acting methylphenidate formulations. Journal of Neural Transmission. Abstracts of the 39th International Danube Symposium for Neurological Sciences and Continuing Education and 1st International Congress on ADHD, from Childhood to Adult Disease 2007;114(7):LXXXIX.
Symons 2007 {published data only}
-
- Symons FJ, Tervo RC, Kim O, Hoch J. The effects of methylphenidate on the classroom behavior of elementary school‐age children with cerebral palsy: a preliminary observational analysis. Journal of Child Neurology 2007;22(1):89‐94. - PubMed
Szobot 2004 {published data only}
-
- Szobot CM, Ketzer C, Parente MA, Biederman J, Rohde LA. The acute effect of methylphenidate in Brazilian male children and adolescents with ADHD: a randomized clinical trial. Journal of Attention Disorders 2004;8(2):37‐43. - PubMed
Szobot 2008 {published data only}
-
- Szobot CM, Rohde LA, Katz B, Ruaro P, Schaefer T, Walcher M, et al. A randomized crossover clinical study showing that methylphenidate‐SODAS improves attention‐deficit/hyperactivity disorder symptoms in adolescents with substance use disorder. Brazilian Journal of Medical and Biological Research 2008;41(3):250‐7. - PubMed
Tannock 1989 {published data only}
-
- Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. Journal of Abnormal Child Psychology 1989;17(5):473‐91. Erratum in: Journal of Abnormal Child Psychology 1990;18(1):119. - PubMed
-
- Tannock R, Schachar RJ, Carr RP, Logan GD. Dose‐response effects of methylphenidate on academic performance and overt behavior in hyperactive children. Pediatrics 1989;84(4):648‐57. - PubMed
Tannock 1992 {published data only}
-
- Tannock R, Schachar R. Methylphenidate and cognitive perseveration in hyperactive children. Journal of Child Psychology and Psychiatry and Allied Disciplines 1992;33(7):1217‐28. - PubMed
Tannock 1993 {published data only}
-
- Tannock R, Schachar RJ, Logan GD. Does methylphenidate induce overfocusing in hyperactive children?. Journal of Clinical Child Psychology 1993;22(1):28‐41.
Tannock 1995a {published data only}
-
- Tannock R, Schachar R, Logan G. Methylphenidate and cognitive flexibility: dissociated dose effects in hyperactive children. Journal of Abnormal Child Psychology 1995;23(2):235‐66. - PubMed
Tannock 1995b {published data only}
-
- Tannock R, Fine J, Heintz T, Schachar RJ. A linguistic approach detects stimulant effects in two children with attention‐deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1995;5(3):177‐89.
-
- Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without comorbid anxiety. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(7):886‐96. - PubMed
Taylor 1987 {published data only}
-
- Taylor E, Schachar R, Thorley G, Wieselberg HM, Everitt B, Rutter M. Which boys respond to stimulant medication? A controlled trial of methylphenidate in boys with disruptive behaviour. Psychological Medicine 1987;17(1):121‐43. - PubMed
Taylor 1993 {published data only}
-
- Taylor MJ, Voros JG, Logan WJ, Malone MA. Changes in event‐related potentials with stimulant medication in children with attention deficit hyperactivity disorder. Biological Psychology 1993;36(3):139‐56. - PubMed
Tervo 2002 {published data only}
-
- Tervo RC, Azuma S, Fogas B, Fiechtner H. Children with ADHD and motor dysfunction compared with children with ADHD only. Developmental Medicine & Child Neurology 2002;44(6):383‐90. - PubMed
Tirosh 1993a {published data only}
-
- Tirosh E, Elhasid R, Kamah SC, Cohen A. Predictive value of placebo methylphenidate. Pediatric Neurology 1993;9(2):131‐3. - PubMed
Tirosh 1993b {published data only}
-
- Tirosh E, Sadeh A, Munvez R, Lavie P. Effects of methylphenidate on sleep in children with attention‐deficient hyperactivity disorder. An activity monitor study. American Journal of Diseases of Children 1993;147(12):1313‐5. - PubMed
Tourette's Syndrome Study Group 2002 {published data only}
-
- Deputy SR. Treatment of ADHD in children with tics: a randomized controlled trial. Clinical Pediatrics 2002;41(9):736. - PubMed
-
- Kurlan R, Goldberg J. Clonidine and methylphenidate were effective for attention deficit hyperactivity disorder in children with comorbid tics. Evidence‐Based Medicine 2002;7(5):157. - PubMed
-
- Kurlan R, Tourette Syndrome Study Group. Treatment of attention‐deficit‐hyperactivity disorder in children with Tourette's syndrome (TACT Trial). Annals of Neurology. Abstracts from the American Neurological Association's 125th Annual Meeting; 2000 October 15‐18; Boston, Massachusetts 2000;48(6):953.
-
- Tourette's Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology 2002;58(4):527‐36. - PubMed
Tucker 2009 {published data only}
-
- Tucker JD, Suter W, Petibone DM, Thomas RA, Bailey NL, Zhou Y, et al. Cytogenetic assessment of methylphenidate treatment in pediatric patients treated for attention deficit hyperactivity disorder. Mutation Research 2009;677(1‐2):53‐8. - PubMed
-
- Zhou Y, Muni R, Tucke JD, Kumar V. Extended‐release methylphenidate exposure and the frequency of cytogenetic abnormalities in children with attention‐deficit‐hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology. Proceedings of the 49th Annual National Institute of Mental Health (NIMH) New Clinical Drug Evaluation Unit (NCDEU) Meeting; 2009 June 29‐ July 2; Hollywood, Florida 2009;19(6):785.
Ullmann 1985 {published data only}
Ullmann 1986 {published data only}
-
- Ullmann RK, Sleator EK. Responders, nonresponders, and placebo responders among children with attention deficit disorder: importance of a blinded placebo evaluation. Clinical Pediatrics 1986;25(12):594‐9. - PubMed
Urman 1995 {published data only}
-
- Urman R, Ickowicz A, Fulford P, Tannock R. An exaggerated cardiovascular response to methylphenidate in ADHD children with anxiety. Journal of Child and Adolescent Psychopharmacology 1995;5(1):29‐37.
Van der Meere 1999a {published data only}
-
- Gunning WB. A Controlled Trial of Clonidine in Hyperkinetic Children. Erasmus Universiteit Rotterdam, 1992.
-
- Meere J, Gunning B, Stemerdink N. The effect of methylphenidate and clonidine on response inhibition and state regulation in children with ADHD. Journal of Child Psychology and Psychiatry and Allied Disciplines 1999;40(2):291‐8. - PubMed
Wallace 1994 {published data only}
-
- Wallace AE, Kofoed LL. Statistical analysis of single case studies in the clinical setting: the example of methylphenidate trials in children with attention‐deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1994;4(3):141‐50.
Wallander 1987 {published data only}
-
- Wallander JL, Schroeder SR, Michelli JA, Gualtieri CT. Classroom social interactions of attention deficit disorder with hyperactivity children as a function of stimulant medication. Journal of Pediatric Psychology 1987;12(1):61‐76. - PubMed
Waxmonsky 2008 {published data only}
-
- Waxmonsky J, Pelham WE, Gnagy E, Cummings MR, O'Connor B, Majumdar A, et al. The efficacy and tolerability of methylphenidate and behavior modification in children with attention‐deficit/hyperactivity disorder and severe mood dysregulation. Journal of Child and Adolescent Psychopharmacology 2008;18(6):573‐88. - PMC - PubMed
Whalen 1990 {published data only}
-
- Whalen CK, Henker B, Granger DA. Ratings of medication effects in hyperactive children: viable or vulnerable?. Behavioral Assessment 1989;11(2):179‐99.
-
- Whalen CK, Henker B, Granger DA. Social judgment processes in hyperactive boys: effects of methylphenidate and comparisons with normal peers. Journal of Abnormal Child Psychology 1990;18(3):297‐316. - PubMed
-
- Whalen CK, Henker B, Hinshaw SP, Granger DA. Externalizing behavior disorders, situational generality, and the type A behavior pattern. Child Development 1989;60(6):1453‐62. - PubMed
Wigal 2003 {published data only}
-
- Wigal SB, Sanchez DY, DeCory HH, D'Imperio JM, Swanson JM. Selection of the optimal dose ratio for a controlled‐delivery formulation of methylphenidate. Journal of Applied Research 2003;3(1):46‐63.
Wigal 2004 {published data only}
-
- Wigal S, Swanson JM, Feifel D, Sangal RB, Elia J, Casat CD, et al. A double‐blind, placebo‐controlled trial of dexmethylphenidate hydrochloride and d,l‐threo‐methylphenidate hydrochloride in children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(11):1406‐14. - PubMed
Wigal 2013 {published data only}
-
- Liquid version of methylphenidate shows efficacy in school trial. The Brown University Child & Adolescent Psychopharmacology Update 2013; Vol. 15, issue 3:1‐3.
-
- Wigal SB, Childress AC, Belden HW, Berry SA. NWP06, an extended‐release oral suspension of methylphenidate, improved attention‐deficit/hyperactivity disorder symptoms compared with placebo in a laboratory classroom study. Journal of Child and Adolescent Psychopharmacology 2013;23(1):3‐10. - PMC - PubMed
Wigal 2014 {published data only}
-
- Wigal SB, Greenhill LL, Nordbrock E, Connor DF, Kollins SH, Adjei A, et al. A randomized placebo‐controlled double‐blind study evaluating the time course of response to methylphenidate hydrochloride extended‐release capsules in children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2014;24(10):562‐9. - PMC - PubMed
Wilens 2006b {published data only}
-
- Oral system methylphenidate for teen ADHD. The Brown University Child & Adolescent Psychopharmacology Update 2006; Vol. 8, issue 3:4‐5.
-
- Biederman J. P.6.053 Effectiveness and safety of the once‐daily OROS formulation of methylphenidate in adolescents with attention‐deficit/hyperactivity disorder. European Neuropsychopharmacology 2003;13(Suppl 4):S448.
-
- Greenhill LL. Safety and efficacy of OROS MPH in adolescents with ADHD. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, CA. San Francisco, 2003:16.
-
- McGough JJ, McBurnett K, Bukstein O, Wilens TE, Greenhill L, Lerner M, et al. Once‐daily OROS methylphenidate is safe and well tolerated in adolescents with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2006;16(3):351‐6. - PubMed
-
- Newcorn JH, Stein MA, Cooper KM. Dose‐response characteristics in adolescents with attention‐deficit/hyperactivity disorder treated with OROS methylphenidate in a 4‐week, open‐label, dose‐titration study. Journal of Child and Adolescent Psychopharmacology 2010;20(3):187‐96. - PubMed
Wilens 2008 {published data only}
-
- López FA, Landgraf JM, Wilens TE. Quality of life and parent satisfaction with the methylphenidate transdermal system. European Neuropsychopharmacology. Papers of the 21st ECNP Congress; 2008 August 30 ‐ September 3; Barcelona, Spain. 2008; Vol. Suppl 4:S562.
-
- López FA, Wilens TE, Wigal SB, Turnbow JM. Effects of variable wear times on transdermal methylphenidate in attention‐deficit/hyperactivity disorder. European Neuropsychopharmacology. Papers of the 21st ECNP Congress; 2008 August 30 ‐ September 3; Barcelona, Spain. 2008; Vol. 18:S561‐2.
-
- Manos M, Frazier TW, Landgraf JM, Weiss M, Hodgkins P. HRQL and medication satisfaction in children with ADHD treated with the methylphenidate transdermal system. Current Medical Research and Opinion 2009;25(12):3001‐10. - PubMed
-
- Wilens TE, Boellner SW, López FA, Turnbow JM, Wigal SB, Childress AC, et al. Varying the wear time of the methylphenidate transdermal system in children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(6):700‐8. - PubMed
Wilens 2010 {published data only}
-
- Wilens T, Hammerness P, Utzinger L, Georgiopoulos A, Doyle R, Brodziak K, et al. Before‐school ADHD symptoms and functioning in youth treated with the Methylphenidate Transdermal Patch (MTS). Journal of Child and Adolescent Psychopharmacology. Abstracts of the 49th Annual National Institute of Mental Health (NIMH) New Clinical Drug Evaluation Unit (NCDEU) Meeting; 2009; June 29 ‐ July 2; Hollywood, Florida. 2009; Vol. 19, issue 6:785‐6.
-
- Wilens TE, Hammerness P, Martelon MK, Brodziak K, Utzinger L, Wong P. A controlled trial of the methylphenidate transdermal system on before‐school functioning in children with attention‐deficit/hyperactivity disorder. Journal of Clinical Psychiatry 2010;71(5):548‐56. - PubMed
Wilkison 1995 {published data only}
-
- Wilkison PC, Kircher JC, McMahon WM, Sloane HN. Effects of methylphenidate on reward strength in boys with attention‐deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(7):897‐901. - PubMed
Wodrich 1998 {published data only}
-
- Wodrich DL, Kush JC. The effect of methylphenidate on teachers' behavioral ratings in specific school situations. Psychology in the Schools 1998;35(1):81‐8.
Wolraich 2001 {published data only}
-
- Greenhill LL. Efficacy and safety of once‐daily methylphenidate HCl, standard methylphenidate and placebo in children with ADHD. Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18; Chicago, Illinois. Chicago, 2000:NR. 667.
-
- Greenhill LL. Evaluation of the efficacy and safety of Concerta (Methylphenidate HCI) extended‐release tablets, Ritalin, and placebo in children with ADHD. Neurology 2000;54(7):A420‐1. [WOS: 000086557801120]
-
- Swanson J, Greenhill L, Pelham W, Wilens T, Wolraich M, Abikoff H, et al. Initiating Concerta (TM) (OROS methylphenidate HCl) qd in children with attention‐deficit hyperactivity disorder. Journal of Clinical Research 2000;3:59‐76.
-
- Wolraich ML. [Efficacy and safety of OROS(R) methylphenidate HCl (MPH) extended‐release tablets (CONCERTA (TM)), conventional MPH, and placebo in children with ADHD]. International Journal of Neuropsychopharmacology. Proceedings of the XXIIst CINP Congress; 2000 July 9‐13; Brussels, Belgium. 2000; Vol. 3 Suppl 1:S329.
-
- Wolraich ML. Efficacy and safety of OROS methylphenidate HCI (MPH) extended‐release tablets (Concerta TM), conventional MPH, and placebo in children with ADHD. International Journal of Neuropsychopharmacology. XXIIst CINP Congress 2000;3(Suppl S1):329.
Zeiner 1999 {published data only}
-
- Zeiner P. Body growth and cardiovascular function after extended treatment (1.75 years) with methylphenidate in boys with attention‐deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1995;5(2):129‐38.
-
- Zeiner P. Do the beneficial effects of extended methylphenidate treatment in boys with attention‐deficit hyperactivity disorder dissipate rapidly during placebo treatment?. Nordic Journal of Psychiatry 1999;53(1):55‐60.
-
- Zeiner P, Bryhn G, Bjercke C, Truyen K, Strand G. Response to methylphenidate in boys with attention‐deficit hyperactivity disorder. Acta Paediatrica 1999;88(3):298‐303. - PubMed
Zeni 2009 {published data only}
-
- Zeni CP, Tramontina S, Ketzer CR, Pheula GF, Rohde LA. Methylphenidate combined with aripiprazole in children and adolescents with bipolar disorder and attention‐deficit/hyperactivity disorder: a randomized crossover trial. Journal of Child and Adolescent Psychopharmacology 2009;19(5):553‐61. - PubMed
References to studies excluded from this review
An 2013 {published data only}
Anderson 2002 {published data only}
-
- Anderson CM, Polcari A, Lowen SB, Renshaw PF, Teicher MH. Effects of methylphenidate on functional magnetic resonance relaxometry of the cerebellar vermis in boys with ADHD. American Journal of Psychiatry 2002;159(8):1322‐8. - PubMed
Barkley 1988a {published data only}
-
- Barkley RA, Fischer M, Newby RF, Breen MJ. Development of a multimethod clinical protocol for assessing stimulant drug response in children with attention deficit disorder. Journal of Clinical Child Psychology 1988;17(1):14‐24.
Barkley 1997 {published data only}
-
- Barkley RA, Koplowitz S, Anderson T, McMurray MB. Sense of time in children with ADHD: effects of duration, distraction, and stimulant medication. Journal of the International Neuropsychological Society 1997;3(4):359‐69. - PubMed
Bart 2013 {published data only}
-
- Bart O, Daniel L, Dan O, Bar‐Haim Y. Influence of methylphenidate on motor performance and attention in children with developmental coordination disorder and attention deficit hyperactive disorder. Research in Developmental Disabilities 2013;34(6):1922‐7. [PUBMED: 23584172] - PubMed
-
- Bart O, Podoly T, Bar‐Haim Y. A preliminary study on the effect of methylphenidate on motor performance in children with comorbid DCD and ADHD. Research in Developmental Disabilities 2010;31(6):1443‐7. [PUBMED: 20650602] - PubMed
Bedard 2002 {published data only}
-
- Bedard A‐C, Ickowicz A, Tannock R. Methylphenidate improves Stroop naming speed, but not response interference, in children with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2002;12(4):301‐9. - PubMed
Bedard 2003 {published data only}
-
- Bedard A‐C, Ickowicz A, Logan GD, Hogg‐Johnson S, Schachar R, Tannock R. Selective inhibition in children with attention‐deficit hyperactivity disorder off and on stimulant medication. Journal of Abnormal Child Psychology 2003;31(3):315‐27. - PubMed
Bedard 2004 {published data only}
-
- Bedard A‐C, Martinussen R, Ickowicz A, Tannock R. Methylphenidate improves visual‐spatial memory in children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(3):260‐8. - PubMed
Bedard 2007 {published data only}
-
- Bedard A‐C, Jain U, Hogg Johnson S, Tannock R. Effects of methylphenidate on working memory components: influence of measurement. Journal of Child Psychology and Psychiatry 2007;48(9):872‐80. - PubMed
Beery 1994 {published data only}
-
- Beery SH. Behavioral disinhibition, anxiety, and response to methylphenidate and behavior management in children with attention deficit hyperactivity disorder. Dissertation Abstracts International: Section B: The Sciences and Engineering 1994;56(2‐B):1096.
Ben‐Pazi 2006 {published data only}
-
- Ben‐Pazi H, Shalev RS, Gross‐Tsur V, Bergman H. Age and medication effects on rhythmic responses in ADHD: possible oscillatory mechanisms?. Neuropsychologia 2006;44(3):412‐6. - PubMed
Bental 2008 {published data only}
-
- Bental B, Tirosh E. The effects of methylphenidate on word decoding accuracy in boys with attention‐deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology 2008;28(1):89‐92. - PubMed
Brown 1984b {published data only}
-
- Brown RT, Wynne ME. Sustained attention in boys with attention deficit disorder and the effect of methylphenidate. Pediatric Nursing 1984;10(1):35‐9. - PubMed
Buhrmester 1992 {published data only}
-
- Buhrmester D, Whalen CK, Henker B, MacDonald V, Hinshaw SP. Prosocial behavior in hyperactive boys: effects of stimulant medication and comparison with normal boys. Journal of Abnormal Child Psychology 1992;20(1):103‐21. - PubMed
Campbell 1996 {published data only}
-
- Campbell L, Malone MA, Kershner JR, Roberts W, Humphries T, Logan WJ. Methylphenidate slows right hemisphere processing in children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1996;6(4):229‐39. - PubMed
Carlson 1991 {published data only}
-
- Carlson CL, Pelham WE Jr, Swanson JM, Wagner JL. A divided attention analysis of the effects of methylphenidate on the arithmetic performance of children with attention‐deficit hyperactivity disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines 1991;32(3):463‐71. - PubMed
Carlson 1992 {published data only}
-
- Carlson CL, Pelham WE Jr, Milich R, Dixon J. Single and combined effects of methylphenidate and behavior therapy on the classroom performance of children with attention‐deficit hyperactivity disorder. Journal of Abnormal Child Psychology 1992;20(2):213‐32. - PubMed
Cox 2004b {published data only}
-
- Cox DJ, Humphrey JW, Merkel RL, Penberthy JK, Kovatchev B. Controlled‐release methylphenidate improves attention during on‐road driving by adolescents with attention‐deficit/hyperactivity disorder. Journal of the American Board of Family Medicine 2004;17(4):235‐9. [DOI: 10.3122/jabfm.17.4.235] - DOI - PubMed
-
- Cox DJ, Merkel RL, Penberthy JK, Kovatchev B, Hankin CS. Impact of methylphenidate delivery profiles on driving performance of adolescents with attention‐deficit/hyperactivity disorder: a pilot study. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(3):269‐75. - PubMed
Dawson 1998 {published data only}
-
- Dawson NL. Effects of methylphenidate on neuropsychological functions myelomeningocele. Dissertation Abstracts International: Section B: The Sciences and Engineering. US: Illinois Inst of Technology, 1998, issue 12‐B:6805.
De Sonneville 1991 {published data only}
-
- Sonneville LM, Njiokiktjien C, Hilhorst RC. Methylphenidate‐induced changes in ADDH information processors. Journal of Child Psychology and Psychiatry and Allied Disciplines 1991;32(2):285‐95. - PubMed
DeVito 2008 {published data only}
Evans 1986 {published data only}
-
- Evans RW, Gualtieri CT, Amara I. Methylphenidate and memory: dissociated effects in hyperactive children. Psychopharmacology 1986;90(2):211‐6. - PubMed
Fox 2014 {published data only}
-
- Fox O, Adi‐Japha E, Karni A. The effect of a skipped dose (placebo) of methylphenidate on the learning and retention of a motor skill in adolescents with attention deficit hyperactivity disorder. European Neuropsychopharmacology 2014;24(3):391‐6. - PubMed
Francis 2001 {published data only}
-
- Francis S, Fine J, Tannock R. Methylphenidate selectively improves story retelling in children with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2001;11(3):217‐28. - PubMed
Gan 1982 {published data only}
-
- Gan J, Cantwell DP. Dosage effects of methylphenidate on paired associate learning: positive/negative placebo responders. Journal of the American Academy of Child Psychiatry 1982;21(3):237‐42. - PubMed
Granger 1996 {published data only}
-
- Granger DA, Whalen CK, Henker B, Cantwell C. ADHD boys' behavior during structured classroom social activities: effects of social demands, teacher proximity, and methylphenidate. Journal of Attention Disorders 1996;1(1):16‐30.
Grizenko 2010 {published data only}
-
- Grizenko N, Paci M, Joober R. Is the inattentive subtype of ADHD different from the combined/hyperactive subtype?. Journal of Attention Disorders 2010;13(6):649‐57. - PubMed
Günther 2010 {published data only}
-
- Günther T, Herpertz‐Dahlmann B, Konrad K. Sex differences in attentional performance and their modulation by methylphenidate in children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2010;20(3):179‐86. - PubMed
Halliday 1983 {published data only}
-
- Halliday R, Callaway E, Naylor H. Visual evoked potential changes induced by methylphenidate in hyperactive children: dose/response effects. Electroencephalography and Clinical Neurophysiology 1983;55(3):258‐67. - PubMed
Hanisch 2004 {published data only}
-
- Hanisch C, Konrad K, Günther T, Herpertz‐Dahlmann B. Age‐dependent neuropsychological deficits and effects of methylphenidate in children with attention‐deficit/hyperactivity disorder: a comparison of pre‐ and grade‐school children. Journal of Neural Transmission 2004;111(7):865‐81. - PubMed
Hazel‐Fernandez 2006 {published data only}
-
- Hazel‐Fernandez LA, Klorman R, Wallace JM, Cook S. Methylphenidate improves aspects of executive function in African American children with ADHD. Journal of Attention Disorders 2006;9(4):582‐9. - PubMed
Hinshaw 1989 {published data only}
-
- Hinshaw SP, Buhrmester D, Heller T. Anger control in response to verbal provocation: effects of stimulant medication for boys with ADHD. Journal of Abnormal Child Psychology 1989;17(4):393‐407. [PUBMED: 2677081] - PubMed
Hinshaw 1993 {published data only}
-
- Hinshaw SP, Heller T, McHale JP. Covert antisocial behavior in boys with attention‐deficit hyperactivity disorder: external validation and effects of methylphenidate. Journal of Consulting and Clinical Psychology 1992;60(2):274‐81. - PubMed
Humphries 1979 {published data only}
-
- Humphries T, Swanson J, Kinsbourne M, Yiu L. Stimulant effects on persistence of motor performance of hyperactive children. Journal of Pediatric Psychology 1979;4(1):55‐66.
King 2009a {published data only}
-
- King S, Waschbusch DA, Pelham WE, Frankland BW, Corkum PV, Jacques S. Subtypes of aggression in children with attention deficit hyperactivity disorder: medication effects and comparison with typical children. Journal of Clinical Child and Adolescent Psychology 2009;38(5):619‐29. - PubMed
King 2009b {published data only}
-
- King S, Waschbusch DA, Pelham WE Jr, Frankland BW, Andrade BF, Jacques S, et al. Social information processing in elementary‐school aged children with ADHD: medication effects and comparisons with typical children. Journal of Abnormal Child Psychology 2009;37(4):579‐89. - PubMed
Lange 2007 {published data only}
-
- Lange KW, Tucha LT, Walitza S, Sontag TA, Stasik D, Linder M, et al. Effects of methylphenidate on multiple components of attention in children with attention deficit hyperactivity disorder. Journal of Neural Transmission. Abstracts of the 39th International Danube Symposium for Neurological Sciences and Continuing Education and 1st International Congress on ADHD, from Childhood to Adult Disease; 2007 June 2‐5; Wurzburg, Germany. 2007; Vol. 114 (7):LXXV.
Leitner 2007b {published data only}
-
- Leitner Y, Barak R, Giladi N, Peretz C, Eshel R, Gruendlinger L, et al. Gait in attention deficit hyperactivity disorder: effects of methylphenidate and dual tasking. Journal of Neurology 2007;254(10):1330‐8. [PUBMED: 17401735] - PubMed
-
- Leitner Y, Doniger GM, Barak R, Simon ES, Hausdorff JM. A novel multidomain computerized cognitive assessment for attention‐deficit hyperactivity disorder: evidence for widespread and circumscribed cognitive deficits. Journal of Child Neurology 2007;22(3):264‐76. [PUBMED: 17621495 ] - PubMed
Malone 1988 {published data only}
-
- Malone MA, Kershner JR, Siegel L. The effects of methylphenidate on levels of processing and laterality in children with attention deficit disorder. Journal of Abnormal Child Psychology 1988;16(4):379‐95. - PubMed
Malone 1993 {published data only}
-
- Malone MA, Swanson JM. Effects of methylphenidate on impulsive responding in children with attention‐deficit hyperactivity disorder. Journal of Child Neurology 1993;8(2):157‐63. - PubMed
Malone 1994 {published data only}
-
- Malone MA, Couitis J, Kershner JR, Logan WJ. Right hemisphere dysfunction and methylphenidate effects in children with attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1994;4(4):245‐53.
Martin 2007 {published data only}
Mehta 2004 {published data only}
-
- Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set‐shifting in AD/HD: relationships to baseline memory capacity. Journal of Child Psychology and Psychiatry and Alllied Disciplines 2004;45(2):293‐305. - PubMed
Milich 1989 {published data only}
-
- Milich R, Licht BG, Murphy DA, Pelham WE. Attention‐deficit hyperactivity disordered boys' evaluations of and attributions for task performance on medication versus placebo. Journal of Abnormal Psychology 1989;98(3):280‐4. - PubMed
Milich 1991 {published data only}
-
- Milich R, Carlson CL, Pelham WE, Licht BG. Effects of methylphenidate on the persistence of ADHD boys following failure experiences. Journal of Abnormal Child Psychology 1991;19(5):519‐36. - PubMed
Novak 1995 {published data only}
-
- Novak GP, Solanto M, Abikoff H. Spatial orienting and focused attention in attention deficit hyperactivity disorder. Psychophysiology 1995;32(6):546‐59. - PubMed
O'Toole 1997 {published data only}
-
- O'Toole K, Abramowitz A, Morris R, Dulcan M. Effects of methylphenidate on attention and nonverbal learning in children with attention‐deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(4):531‐8. - PubMed
Peeke 1984 {published data only}
-
- Peeke S, Halliday R, Callaway E, Prael R, Reus V. Effects of two doses of methylphenidate on verbal information processing in hyperactive children. Journal of Clinical Psychopharmacology 1984;4(2):82‐8. - PubMed
Pelham 1985 {published data only}
-
- Pelham WE, Bender ME, Caddell J, Booth S, Moorer SH. Methylphenidate and children with attention deficit disorder. Dose effects on classroom academic and social behavior. Archives of General Psychiatry 1985;42(10):948‐52. - PubMed
Pelham 1990b {published data only}
-
- Pelham WE, McBurnett K, Harper GW, Milich R, Murphy DA, Clinton J, et al. Methylphenidate and baseball playing in ADHD children: who's on first?. Journal of Consulting and Clinical Psychology 1990;58(1):130‐3. [PUBMED: 2319047] - PubMed
Pelham 1992 {published data only}
-
- Pelham WE, Murphy DA, Vannatta K, Milich R. Methylphenidate and attributions in boys with attention‐deficit hyperactivity disorder. Annual Progress in Child Psychiatry & Child Development. American Psychological Association, 1993:242‐65.
-
- Pelham WE, Murphy DA, Vannatta K, Milich R, Licht BG, Gnagy EM, et al. Methylphenidate and attributions in boys with attention‐deficit hyperactivity disorder. Journal of Consulting and Clinical Psychology 1992;60(2):282‐92. [PsycINFO: 1994‐34504‐001] - PubMed
Pelham 1997 {published data only}
-
- Pelham WE, Hoza B, Kipp HL, Gnagy EM, Trane ST. Effects of methylphenidate and expectancy on ADHD children's performance, self‐evaluations, persistence, and attributions on a cognitive task. Experimental and Clinical Psychopharmacology 1997;5(1):3‐13. - PubMed
Pelham 2001b {published data only}
-
- Pelham WE Jr, Waschbusch DA, Hoza B, Pillow DR, Gnagy EM. Effects of methylphenidate and expectancy on performance, self‐evaluations, persistence, and attributions on a social task in boys with ADHD. Experimental and Clinical Psychopharmacology 2001;9(4):425‐37. - PubMed
Rapport 1995 {published data only}
-
- Rapport MD, Loo S, Denney C. The paired associate learning task: is it an externally valid instrument for assessing methylphenidate response in children with attention deficit disorder?. Journal of Psychopathology and Behavioral Assessment 1995;17(2):125‐44.
Richardson 1988 {published data only}
-
- Kupietz SS, Winsberg BG, Sverd J. Learning ability and methylphenidate (Ritalin(®)) plasma concentration in hyperkinetic children: a preliminary investigation. Journal of the American Academy of Child and Adolescent Psychiatry 1982;21(1):27‐30. - PubMed
-
- Richardson E, Kupietz SS, Winsberg BG, Maitinsky S, Mendell N. Effects of methylphenidate dosage in hyperactive reading‐disabled children: II. Reading achievement. Journal of the American Academy of Child and Adolescent Psychiatry 1988;27(1):78‐87. - PubMed
Rubia 2003 {published data only}
-
- Rubia K, Noorloos J, Smith A, Gunning B, Sergeant J. Motor timing deficits in community and clinical boys with hyperactive behavior: the effect of methylphenidate on motor timing. Journal of Abnormal Child Psychology 2003;31(3):301‐13. - PubMed
Sangal 2006 {published data only}
-
- Sangal RB, Sangal JM. Attention‐deficit/hyperactivity disorder: use of cognitive evoked potential (P300) to predict treatment response. Clinical Neurophysiology 2006;117(9):1996‐2006. - PubMed
Sengupta 2008 {published data only}
Silk 2012 {published data only}
-
- Silk T. Resting‐state functional connectivity anomalies in ADHD and responses to methylphenidate medication. Brain Connectivity 2012; Vol. 2, issue 4:A128.
Smith 2013 {published data only}
-
- Smith A, Cubillo A, Barrett N, Giampietro V, Simmons A, Brammer M, et al. Neurofunctional effects of methylphenidate and atomoxetine in boys with attention‐deficit/hyperactivity disorder during time discrimination. Biological Psychiatry 2013;74(8):615‐22. - PubMed
Solanto 1986 {published data only}
-
- Solanto MV. Behavioral effects of low‐dose methylphenidate in childhood attention deficit disorder: implications for a mechanism of stimulant drug action. Journal of the American Academy of Child and Adolescent Psychiatry 1986;25(1):96‐101. - PubMed
Solanto 1997 {published data only}
-
- Solanto MV, Wender EH, Bartell SS. Effects of methylphenidate and behavioral contingencies on sustained attention in attention‐deficit hyperactivity disorder: a test of the reward dysfunction hypothesis. Journal of Child and Adolescent Psychopharmacology 1997;7(2):123‐36. - PubMed
Srinivas 1992 {published data only}
-
- Srinivas NR, Hubbard JW, Quinn D, Midha KK. Enantioselective pharmacokinetics and pharmacodynamics of dl‐threo‐methylphenidate in children with attention deficit hyperactivity disorder. Clinical Pharmacology and Therapeutics 1992;52(5):561‐8. - PubMed
Strand 2012 {published data only}
-
- Strand MT, Hawk LW Jr, Bubnik M, Shiels K, Pelham WE Jr, Waxmonsky JG. Improving working memory in children with attention‐deficit/hyperactivity disorder: the separate and combined effects of incentives and stimulant medication. Journal of Abnormal Child Psychology 2012; Vol. 40, issue 7:1193‐207. - PMC - PubMed
Stray 2009 {published data only}
Swanson 1993 {published data only}
-
- Swanson J, McBurnett K, Wigal T, Pfiffner LJ. Effect of stimulant medication on children with attention deficit disorder: a "review of reviews". Exceptional Children 1993;60(2):154‐61.
Szobot 2003 {published data only}
-
- Szobot CM, Ketzer C, Cunha RD, Parente MA, Langleben DD, Acton PD, et al. The acute effect of methylphenidate on cerebral blood flow in boys with attention‐deficit/hyperactivity disorder. European Journal of Nuclear Medicine and Molecular Imaging 2003;30(3):423‐6. - PubMed
Tannock 2000 {published data only}
-
- Tannock R, Martinussen R, Frijters J. Naming speed performance and stimulant effects indicate effortful, semantic processing deficits in attention‐deficit/hyperactivity disorder. Journal of Abnormal Child Psychology 2000;28(3):237‐52. - PubMed
Teicher 2003 {published data only}
-
- Teicher MH, Polcari A, Anderson CM, Andersen SL, Lowen SB, Navalta CP. Rate dependency revisited: understanding the effects of methylphenidate in children with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2003;13(1):41‐51. - PubMed
Teicher 2006 {published data only}
-
- Teicher MH, Polcari A, Foley M, Valente E, McGreenery CE, Chang WW, et al. Methylphenidate blood levels and therapeutic response in children with attention‐deficit hyperactivity disorder: I. Effects of different dosing regimens. Journal of Child and Adolescent Psychopharmacology 2006;16(4):416‐31. - PubMed
Teicher 2007 {published data only}
-
- Teicher MH, Polcari A, McGreenery CE. Objective measures of activity and attention accurately identify methylphenidate doses associated with optimal clinical response in children with ADHD. Biological Psychiatry 2007;61(8 Suppl):66S.
Tillery 2000 {published data only}
-
- Tillery KL. A double‐blind study of the Central Auditory Processing and Auditory Continuous Test performances of children with attention deficit hyperactivity disorder and central auditory processing disorder under Ritalin and placebo conditions. Dissertation Abstracts International: Section A: Humanities and Social Sciences 1998; Vol. 58, issue 7‐A:2536.
-
- Tillery KL, Katz J, Keller WD. Effects of methylphenidate (Ritalin) on auditory performance in children with attention and auditory processing disorders. Journal of Speech, Language, and Hearing Research 2000;43(4):893‐901. [PUBMED: 11386476] - PubMed
Trommer 1991 {published data only}
-
- Trommer BL, Hoeppner JA, Zecker SG. The go‐no go test in attention deficit disorder is sensitive to methylphenidate. Journal of Child Neurology 1991;6(1 Suppl):S128‐31. - PubMed
Tsang 2012 {published data only}
-
- Tsang TW, Kohn MR, Clarke SD, Williams LM. Cognition and emotion in child and adolescent ADHD. Biological Psychiatry. Proceedings of the 67th Society of Biological Psychiatry Annual Meeting; 2012 May 2‐4. 2012; Vol. 71 (8 Suppl):74S.
Tucha 2006 {published data only}
-
- Tucha O, Prell S, Mecklinger L, Bormann‐Kischkel C, Kübber S, Linder M, et al. Effects of methylphenidate on multiple components of attention in children with attention deficit hyperactivity disorder. Psychopharmacology 2006;185(3):315‐26. - PubMed
Verbaten 1994 {published data only}
-
- Verbaten MN, Overtoom CC, Koelega HS, Swaab‐Barneveld H, Gaag RJ, Buitelaar J, et al. Methylphenidate influences on both early and late ERP waves of ADHD children in a continuous performance test. Journal of Abnormal Child Psychology 1994;22(5):561‐78. - PubMed
Waschbusch 2007 {published data only}
-
- Waschbusch DA, Craig R, Pelham WE Jr, King S. Self‐handicapping prior to academic‐oriented tasks in children with attention deficit/hyperactivity disorder (ADHD): medication effects and comparisons with controls. Journal of Abnormal Child Psychology 2007;35(2):275‐86. - PubMed
Whalen 1987 {published data only}
-
- Whalen CK, Henker B, Swanson JM, Granger D, Kliewer W, Spencer J. Natural social behaviors in hyperactive children: dose effects of methylphenidate. Journal of Consulting and Clinical Psychology 1987;55(2):187‐93. - PubMed
References to studies awaiting assessment
Drtilkova 1997 {published data only}
-
- Drtilkova I, Misurec J, Balastikova B. Methylphenidate, amphetaminil and mesocarb in children with hyperkinetic disorder: predicting value changes the pharmo‐EEG profile of psychostimulatory substances [Metylfenidat, amfetaminil a mesocarb u deti s hyperkinetickou poruchou: prediktivni hodnota zmen farmakologickeho EEG profilu psychostimulacnich latek]. Ceska a Slovenska Psychiatrie 1997;93(Suppl 3):44‐53.
Short 2004 {published data only}
-
- Short EJ, Manos MJ, Findling RL, Schubel EA. A prospective study of stimulant response in preschool children: insights from ROC analyses. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(3):251‐9. - PubMed
References to ongoing studies
Bottelier 2014 {published data only}
Gendron 2012 {published data only}
-
- Gendron M, Rusak B, Rajda M, Corkum PV. Assessing the impact of methylphenidate on sleep in children with ADHD using polysomnography and actigraphy. Sleep 2012;35:A374.
NCT00483106 {unpublished data only}
-
- NCT00483106. Clinical and pharmacological study of attention deficit with hyperactivity disorder (ADHD). https://clinicaltrials.gov/ (accessed 10 March 2014).
NCT02039908 {unpublished data only}
-
- NCT02039908. Examining tolerance to CNS stimulants in ADHD. https://clinicaltrials.gov/ (accessed 4 April 2015).
Nurmi 2013 {published data only}
-
- Nurmi EL, Mallya A, Mallya KS, Hellemann GS, McGough J, Loo SK, et al. Pharmacogenetics of growth effects complicating ADHD treatment. Neuropsychopharmacology. Proceedings of the 52nd Annual Meeting of the American College of Neuropsychopharmacology; 2013 December 8‐12; Hollywood, USA. 2013 (S2); Vol. 38:S492‐3.
-
- Nurmi EL, Mallya KS, McGough J, Loo SK, Bilder RM, Whelan F, et al. Pharmacogenetic moderators of methylphenidate and guanfacine response in children and adolescents with ADHD. Neuropsychopharmacology. Proceedings of the 51st Annual Meeting of the American College of Neuropsychopharmacology; 2012 December 2‐6; Hollywood; USA. 2012; Vol. 38 (S1):S219.
Additional references
Aasberg 1978
-
- Aasberg M, Montgomery SA, Perris C, Schalling D, Sedvall G. A comprehensive psychopathological rating scale. Acta Psychiatrica Scandinavica. Supplementum 1978;57(S271):5‐27. - PubMed
Abikoff 1980
-
- Abikoff H, Gittelman R, Klein DF. Classroom observation code for hyperactive children: a replication of validity. Journal of Consulting and Clinical Psychology 1980;48(5):555‐65. - PubMed
Achenbach 1986
-
- Achenbach TM, Edelbrock CS. Manual for the Teacher's Report Form and Teacher Version of the Child Behavior Profile. Burlington, VT: University of Vermont, Department of Psychiatry, 1986.
Achenbach 1991
-
- Achenbach TM. Integrative Guide to the 1991 CBCL/4‐18, YSR, and TRF Profiles. Burlington, VT: University of Vermont, Department of Psychiatry, 1991.
Achenbach 1991a
-
- Achenbach TM. Manual for the Child Behavior Checklist/4‐18 and 1991 Profile. Burlington, VT: University of Vermont, Department of Pyshciatry, 1991.
Achenbach 1991b
-
- Achenbach TM. Manual for the Teacher Report Form. Burlington, VT: University of Vermont, Department of Psychiatry, 1991.
Achenbach 2001
-
- Achenbach TM, Rescorla LA. Manual for the ASEBA School‐Age Forms and Profiles. Burlington, VT: ASEBA, 2001.
AIM‐C 2013
-
- AIM‐C 2015. ADHD Impact module ‐ child. bit.ly/1fgWlKo (accessed 6 July 2015).
Aman 1996
-
- Aman MG, Tassé MJ, Rojahn J, Hammer D. The Nisonger CBRF: a child behavior rating form for children with developmental disabilities. Research in Developmental Disabilities 1996;17(1):41‐57. - PubMed
American Academy of Pediatrics 2011
-
- Subcommittee on Attention‐Deficit/Hyperactivity Activity Disorder, Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention‐deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011;128(5):1007‐22. - PMC - PubMed
Andrews 2013a
-
- Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck‐Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719–25. - PubMed
Andrews 2013b
-
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation ‐ determinants of a recommendation’s direction and strength. Journal of Clinical Epidemiology 2013;66(7):726–35. - PubMed
Angold 1995
-
- Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The Child and Adolescent Psychiatric Assessment (CAPA). Psychological Medicine 1995;25(4):739‐53. - PubMed
APA 1980
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III). 3rd Edition. Washington, DC: American Psychiatric Association, 1980.
APA 1987
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM III‐R). 3rd Edition. Washington, DC: American Psychiatric Association, 1987.
APA 1994
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington, DC: American Psychiatric Association, 1994.
APA 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR) Text revision. 4th Edition. Washington, DC: American Psychiatric Publishing, 2000.
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). 5th Edition. Washington, DC: American Psyciatric Association, 2013.
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401–6. - PubMed
Barkley 1977a
-
- Barkley RA. A review of stimulant drug research with hyperactive children. Journal of Child Psychology and Psychiatry 1977;18(2):137‐65. - PubMed
Barkley 1981
-
- Barkley RA. The use of psychopharmacology to study reciprocal influences in parent‐child interaction. Journal of Abnormal Child Psychology 1981;9(3):303‐10. - PubMed
Barkley 1987
-
- Barkley RA, Edelbrock CS. In: Prinz R editor(s). Assessing Situational Variation in Children's Behavior Problems: The Home and School Situations Questionnaires. Greenwich, CT: JAI Press, 1987.
Barkley 1988b
-
- Barkley RA. Child behavior rating scales and checklists. In: Rutter M, Tuma AH, Lann I editor(s). Assessment and Diagnosis in Child Psychopathology. New York: Guilford Press, 1988:113‐55.
Barkley 1989a
-
- Barkley RA. Hyperactive girls and boys: stimulant drug effects on mother‐child interactions. Journal of Child Psychology and Psychiatry 1989;30(3):379‐90. - PubMed
Barkley 1990
-
- Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo‐controlled evaluation. Pediatrics 1990;86(2):184‐92. - PubMed
Barkley 1991a
-
- Barkley RA. Attention‐Deficit Hyperactivity Disorder: A Clinical Workbook. New York: Guilford Press, 1991.
Barkley 1991b
-
- Barkley RA, DuPaul GJ, McMurray MB. Attention deficit disorder with and without hyperactivity: clinical response to three dose levels of methylphenidate. Pediatrics 1991;87(4):519‐31. - PubMed
Barkley 1998
-
- Barkley RA, Murphy KR, Bauermeister JJ. Attention‐Deficit Hyperactivity Disorder: A Clinical Workbook. 2nd Edition. New York: Guilford Press, 1998.
Becker 1993
Berkey 1996
-
- Berkey CS, Mosteller F, Lau J, Antman E. Uncertainty of the time of first significance in random effects cumulative meta‐analysis. Controlled Clinical Trials 1996;17(5):357‐71. - PubMed
Bero 2013
Biederman 2003b
-
- Biederman J. Pharmacotherapy for attention‐deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow‐up of youths with and without ADHD. Journal of Clinical Psychiatry 2003;64(Suppl 11):3‐8. - PubMed
Bloch 2009
Block 1998
-
- Block SL. Attention‐deficit disorder: a paradigm for psychotropic medication intervention in pediatrics. Pediatric Clinics of North America 1998;45(5):1053‐83. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analysis. Journal of Clinical Epidemiology 2008;61(8):763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analysis may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analysis. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
Brunetti 2013
-
- Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. Journal of Clinical Epidemiology 2013;66(2):140–50. - PubMed
Bruni 1996
-
- Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. Journal of Sleep Research 1996;5(4):251‐61. - PubMed
Bussing 2008
CADDRA 2011
-
- Canadian Attention Deficit Hyperactivity Disorder Resource Alliance. Canadian ADHD Practice Guidelines. 3rd Edition. Toronto: CADDRA, 2011.
Castellanos 2006
-
- Castellanos FX, Sonuga‐Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends in Cognitive Sciences 2006;10(3):117‐23. - PubMed
Caye 2013
Chambers 1985
-
- Chambers WJ, Puig‐Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, et al. The assessment of affective disorders in children and adolescents by semistructured interview. Test‐retest reliability of the schedule for affective disorders and schizophrenia for school‐age children, present episode version. Archives of General Psychiatry 1985;42(7):696‐702. - PubMed
Chan 2013
Charach 2011
-
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention‐deficit/hyperactivity disorder and future substance use disorders: comparative meta‐analyses. Journal of the American Academy of Child and Adolescent Psychiatry 2011;50(1):9‐21. - PubMed
Charach 2013
-
- Charach A, Carson P, Fox S, Ali MU, Beckett J, Lim CG. Interventions for preschool children at high risk for ADHD: a comparative effectiveness review. Pediatrics 2013;131(5):e1584‐604. - PubMed
Chen 2006
-
- Chen W, Taylor E. Parental account of children's symptoms (PACS), ADHD phenotypes and its applications to molecular genetic studies. In: Oades RD editor(s). Attention‐Deficit/Hyperactivity Disorders and the Hyperkinetic Syndrome: Current Ideas and Ways Forward. Hauppage, NY: Nova Science Publishing, 2006:3‐20.
Cherland 1999
-
- Cherland E, Fritzpark R. Psychotic side effects of psychostimulants: a 5‐year review. Canadian Journal of Psychiatry 1999;44(8):811‐3. - PubMed
Chervin 2000
-
- Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep‐disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Medicine 2000;1(1):21‐32. - PubMed
Clark 1993
-
- Clark LA. Manual for the Schedule for Nonadaptive and Adaptive Personality (SNAP): Manual for Administration, Scoring, and Interpretation. Minneapolis: University of Minnesota Press, 1993.
Clark 1996
-
- Clark LA. Schedule for Nonadaptive and Adaptive Personality. Manual for Administration, Scoring, and Interpretation. Minneapolis: University of Minnesota Press, 1996.
Conners 1985
-
- Conners CK, Barkley RA. Rating scales and checklists for child psychopharmacology. Psychopharmacology Bulletin 1985;21(4):809‐43. - PubMed
Conners 1995
-
- Conners CK. The Conners' Parent Rating Scales. Toronto: Multi‐Health Systems, 1995.
Conners 1997a
-
- Conners CK. Conners' Rating Scales. Revised. North Tonowanda: Multi‐Health Systems, 1997.
Conners 1997b
-
- Conners CK, Wells KC, Parker JD, Sitarenios G, Diamond JM, Powell JW. A new self‐report scale for assessment of adolescent psychopathology: factor structure, reliability, validity, and diagnostic sensitivity. Journal of Abnormal Child Psychology 1997;25(6):487‐97. [DOI: 10.1023/A:1022637815797] - DOI - PubMed
Conners 1998a
-
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS‐R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology 1998;26(4):279‐91. - PubMed
Conners 1998b
-
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners' Parent Rating Scale (CPRS‐R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology 1998;26(4):257‐68. - PubMed
Conners 2001
-
- Conners CK. Conners Rating Scales, Revised. North Tonawanda: Multi‐Health Systems, 2001.
Conners 2008
-
- Conners CK. Conners 3rd Edition. Toronto: Multi‐Health Systems Inc, 2008.
Connor 2002
-
- Connor DF. Preschool attention deficit hyperactivity disorder: a review of prevalence, diagnosis, neurobiology, and stimulant treatment. Journal of Developmental and Behavioral Pediatrics 2002;23(1 Suppl):S1‐S9. - PubMed
Corkum 2007
-
- Corkum P, Andreou P, Schachar R, Tannock R, Cunningham C. The Telephone Interview Probe: a novel measure of treatment response in children with attention deficit hyperactivity disorder. Educational and Psychological Measurement 2007;67(1):169‐85.
Cox 2004a
-
- Cox DJ, Merkel RL, Penberthy JK, Kovatchev B, Hankin CS. Impact of methylphenidate delivery profiles on driving performance of adolescents with attention‐deficit/hyperactivity disorder: a pilot study. Journal of the American Acadademy of Child and Adolescent Psychiatry 2004;43(3):269‐75. - PubMed
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ trial sequential analysis. www.ctu.dk/tsa (accessed 4 March 2015).
Curtin 2002
-
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. III: The issue of carry‐over. Statistics in Medicine 2002;21(15):2161‐73. - PubMed
DuPaul 1991a
-
- DuPaul GJ. Parent and teacher ratings of ADHD symptoms: psychometric properties in a community‐based sample. Journal of Clinical Child Psychology 1991;20(3):245‐53.
DuPaul 1992
-
- DuPaul GJ, Barkley RA. Situational variability of attention problems: psychometric properties of the Revised Home and School Situations Questionnaires. Journal of Clinical Child Psychology 1992;21(2):178‐88.
Döpfner 2000
-
- Döpfner M, Lehmkuhl G. Diagnostik‐System für Psychische Störungen im Kindes‐ und Jugendalter nach ICD‐10 und DSM‐IV (DISYPS‐KJ). Bern: Huber, 2000.
Döpfner 2008
-
- Döpfner M, Görtz‐Dorten A, Lehmkuhl G. Diagnostik‐System für Psychische Störungen im Kindes‐ und Jugendalter nach ICD‐10 und DSM‐IV. DISYPS‐II. Bern: Huber, 2008.
Efstratopoulou 2013
-
- Efstratopoulou M, Simons J, Janssen R. Concordance among physical educators’, teachers’, and parents’ perceptions of attention problems in children. Journal of Attention Disorders 2013;17(5):437‐43. - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] - PubMed
Engert 2008
Epstein 2014
Ercan 2001
-
- Ercan ES, Amado S, Somer O, Çıkoğlu S. Development of a test battery for attention deficit hyperactivity and disruptive behavior disorders [Dikkat eksikligi hiperaktivite bozuklugu ve yikici davranis bozukluklari icin bir test bataryasi gelistirme cabasi]. Turkish Journal of Child and Adolescent Mental Health 2001;8(3):132‐44.
Fabiano 2006
-
- Fabiano GA, Pelham WE Jr, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, et al. A practical measure of impairment: psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school‐based samples. Journal of Clinical Child and Adolescent Psychology 2006;35(3):369‐85. - PubMed
Faraone 2002
-
- Faraone SV, Biederman J, Roe C. Comparative efficacy of Adderall and methylphenidate in attention‐deficit/hyperactivity disorder: a meta‐analysis. Journal of Clinical Psychopharmacology 2002;22(5):468‐73. - PubMed
Faraone 2006
Faraone 2009
Faraone 2010
-
- Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention‐deficit/hyperactivity disorder using meta‐analysis of effect sizes. Journal of Clinical Psychiatry 2010;71(6):754‐63. - PubMed
Fischer 1998
-
- Fischer M, Newby RF. Use of the restricted academic task in ADHD dose‐response relationships. Journal of Learning Disabilities 1998;31(6):608‐12. - PubMed
Fish 1985
-
- Fish B. Children's Psychiatric Rating Scale (scoring). Psychopharmacology Bulletin 1985;21(4):1764‐985.
Fitzpatrick 1992b
-
- Fitzpatrick PA, Klorman R, Brumaghim JT, Borgstedt AD. Effects of sustained‐release and standard preparations of methylphenidate on attention deficit disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31(2):226‐34. - PubMed
Gadow 1994
-
- Gadow KD, Sprafkin J. Child Symptom Inventories Manual. Stony Brook, NY: Checkmate Plus, 1994.
Gadow 1996
-
- Gadow KD, Sprafkin J, Nolan EE. ADHD School Observation Code. New York: Checkmate Plus, 1996.
Gale 1986
-
- Gale J, Pfefferbaum B, Suhr MA, Overall JE. The Brief Psychiatric Rating Scale for Children: a reliability study. Journal of Clinical Child Psychology 1986;15(4):341‐5.
Gilmore 2001
-
- Gilmore A, Milne R. Methylphenidate in children with hyperactivity: review and cost‐utility analysis. Pharmacoepidemology and Drug Safety 2001;10(2):85‐94. - PubMed
Gittleman‐Klein 1976
-
- Gittleman‐Klein R, Klein DF. Methylphenidate effects in learning disabilities: psychometric changes. Archives of General Psychiatry 1976;33(6):655‐64. - PubMed
Gluud 2015b
-
- The Nordic Trial Alliance Working Group 6 on Transparency and Registration. Report on transparency and registration in clinical research in the Nordic countries. bit.ly/1S2H1Np (accessed 30 April 2015).
Gould 2009
-
- Gould M S, Walsh B T, Munfakh J L, Kleinman M, Duan N H, Olfson M, et al. Sudden Death and Use of Stimulant Medications in Youths. American Journal of Psychiatry 2009;166(9):992‐1001. - PubMed
Goyette 1978
-
- Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners Parent and Teacher Rating Scales. Journal of Abnormal Child Psychology 1978;6(2):221‐36. - PubMed
GRADEpro 2014 [Computer program]
-
- McMaster Unviersity. GRADEpro. McMaster Unviersity, 2014.
Greenhill 2006b
-
- Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, et al. Efficacy and safety of immediate release methylphenidate treatment for preschoolers with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(11):1284‐93. - PubMed
Guy 1976
-
- Guy W. ECDEU Assessment Manual for Psychopharmacology ‐ Revised (DHHS Publ No ADM 91‐338). Rockville, MD, US: Department of Health and Human Services, 1976:218‐22.
Guy 1976a
-
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration, 1976.
Guyatt 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383–94. - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395–400. - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407–15. - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277–82. - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283–93. - PubMed
Guyatt 2011f
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294–302. - PubMed
Guyatt 2011g
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303–10. - PubMed
Guyatt 2011h
-
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311–6. - PubMed
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151–7. - PubMed
Guyatt 2013b
-
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158–72. - PubMed
Guyatt 2013c
-
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173–83. - PubMed
Gøtzsche 2015
-
- Gøtzsche P. Comments on the editorial by Sterne: Why the Cochrane risk of bias tool should include funding source as a standard item [editorial]. Comment by Peter Gøtzsche 9 January 2014. Cochrane Database of Systematic Reviews 10 March 2015.
Hanwella 2011
Hartman 2007
-
- Hartman CA, Rhee SH, Willcutt EG, Pennington, BF. Modeling rater disagreement for ADHD: are parents or teachers biased?. Journal of Abnormal Child Psychology 2007;35(4):536‐42. [PUBMED: 17333362] - PubMed
Heal 2006
-
- Heal DJ, Pierce DM. Methylphenidate and its isomers: their role in the treatment of attention‐deficit hyperactivity disorder using a transdermal delivery system. CNS Drugs 2006;20(9):713‐38. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Holden 2013
-
- Holden SE, Jenkins‐Jones S, Poole CD, Morgan CL, Coghill D, Currie CJ. The prevalence and incidence, resource use and financial costs of treating people with attention deficit/hyperactivity disorder (ADHD) in the United Kingdom (1998 to 2010). Child and Adolescent Psychiatry and Mental Health 2013;11(7):34. - PMC - PubMed
Hollis 1999
Holmskov 2014 [pers comm]
-
- Holmskov M. Data requested [personal communication]. Email to: Dr Leddy 11 June 2014.
Humphrey 1982
-
- Humphrey LL. Children's and teachers' perspectives on children's self‐control: the development of two rating scales. Journal of Consulting and Clinical Psychology 1982;50(5):624‐33. - PubMed
ICH 1996
-
- ICH Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice E6(R1). http://bit.ly/1B0jeJg (accessed 4 March 2014).
Iversen 2006
Jakobsen 2013
-
- Jakobsen JC, Gluud C. The necessity of randomised clinical trials. British Journal of Medicine and Medical Research 2013;3(4):1453‐68.
Jakobsen 2014
Jensen 2001
-
- Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):147‐58. - PubMed
Kadesjö 2001
-
- Kadesjö B, Gillberg C. The comorbidity of ADHD in the general population of Swedish school‐age children. Journal of Child Psychology and Psychiatry and Allied Disciplines 2001;42(4):487‐92. - PubMed
Kadesjö 2002
-
- Kadesjö B. ADHD in Children and Adults [ADHD hos barn och vuxna]. Stockholm: Socialstyrelsen, 2002.
Kambeitz 2014
-
- Kambeitz J, Romanos M, Ettinger U. Meta‐analysis of the association between dopamine transporter genotype and response to methylphenidate treatment in ADHD. Pharmacogenomics Journal 2014;14(1):77‐84. - PubMed
Kanjwal 2012
-
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Use of methylphenidate in the treatment of patients suffering from refractory postural tachycardia syndrome. American Journal of Therapeutics 2012;19(1):2‐6. - PubMed
Kelsey 2004
-
- Kelsey DK, Sumner CR, Casat CD, Coury DL, Quintana H, Saylor KE, et al. Once‐daily atomoxetine treatment for children with attention‐deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double‐blind, placebo‐controlled trial. Pediatrics 2004;114(1):e1‐8. - PubMed
Kendall 1979
-
- Kendall PC, Wilcox LE. Self‐control in children: development of a rating scale. Journal of Consulting and Clinical Psychology 1979;47(6):1020‐9. - PubMed
Kidd 2000
-
- Kidd PM. Attention deficit/hyperactivity disorder (ADHD) in children: rationale for its integrative management. Alternative Medicine Review 2000;5(5):402‐28. - PubMed
Kieling 2010
Kimko 1999
-
- Kimko HC, Cross JT, Abernethy DR. Pharmacokinetics and clinical effectiveness of methylphenidate. Clinical Pharmacokinetics 1999;37(6):457–70. - PubMed
King 2006
-
- King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al. A systematic review and economic model of the effectiveness and cost‐effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents. Health Technology Assessment. NIHR Health Technology Assessment Programme, 2006; Vol. 10, issue 23:iii‐iv, xiii‐146. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Krogh 2013a [pers comm]
-
- Krogh HB. Requested data from the trial [personal communication]. Email to: Dr Brams 23 September 2013.
Krogh 2013b [pers comm]
-
- Krogh HB. Requestion data on inclusion criteria [personal communication]. Email to: Dr Cox 8 October 2013.
Krogh 2013c [pers comm]
-
- Krogh HB. Data received from author [personal communication]. Email to: Dr Wolraich 2 July 2013.
Krogh 2014a [pers comm]
-
- Krogh HB. Randomisation procedure and analyses used in our trial [personal communication]. Email to: Russel Barkley 15 February 2014.
Krogh 2014b [pers comm]
-
- Krogh BH. Data requested [personal communication]. Email to: Dr Johnson June 2014.
Krogh 2014c [pers comm]
-
- Krogh HB. Data regarding randomisation procedure. Email to: Dr Klorman 21 January 2014.
Lachar 1986
-
- Lachar D, Klein R, Boersma D. Personality Inventory for Children: approaches to actuarial interpretation in clinic and school settings. In: Knoff H editor(s). The Assessment of Child and Adolescent Personality. New York: Guilford Press, 1986:273‐308.
Ladd 1996
-
- Ladd GW, Profilet SM. The Child Behavior Scale: a teacher‐report measure of young children's aggressive, withdrawn, and prosocial behaviors. Developmental Psychology 1996;32(6):1008‐24.
Lahey 2006
-
- Lahey BB, Pelham WE, Chronis A, Massetti G, Kipp H, Ehrhardt A, et al. Predictive validity of ICD‐10 hyperkinetic disorder relative to DSM‐IV attention‐deficit/hyperactivity disorder among younger children. Journal of Child Psychology and Psychiatry and Allied Disciplines 2006;47(5):472‐9. - PubMed
Landgraf 1998
-
- Landgraf JM, Maunsell E, Speechley KN, Bullinger M, Campbell S, Abetz L, et al. Canadian‐French, German and UK versions of the Child Health Questionnaire: methodology and preliminary item scaling results. Quality of Life Research 1998;7(5):433‐45. - PubMed
Landgraf 2002
-
- Landgraf JM, Rich M, Rappaport L. Measuring quality of life in children with attention‐deficit/hyperactivity disorder and their families: development and evaluation of a new tool. Archives of Pediatrics and Adolescent Medicine 2002;156(4):384‐91. - PubMed
Lau 1995
-
- Lau J, Schmid CH, Chalmers TC. Cumulative meta‐analysis of clinical trials builds evidence for exemplary medical care. Journal of Clinical Epidemiology 1995;48(1):45‐57. - PubMed
Lavigne 2012
-
- Lavigne JV, Dulcan MK, LeBailly SA, Binns HJ. Can parent reports serve as a proxy for teacher ratings in medication management of attention‐deficit hyperactivity disorder?. Journal of Developmental & Behavioral Pediatrics 2012;33(4):336‐42. - PubMed
Leckman 1988
-
- Leckman JF, Towbin K, Ort SI. Clinical assessment of tic disorder severity. In: Cohen DJ, Bruun RD, Leckman JF editor(s). Tourette's Syndrome and Tic Disorders: Clinical Understanding and Treatment. Oxford: John Wiley & Sons, 1988:55‐78.
Leckman 1989
-
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: initial testing of a clinician‐rated scale of tic severity. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(4):566‐73. - PubMed
Lee 2008
-
- Lee SI, Schachar RJ, Chen SX, Ornstein TJ, Charach A, Barr C, et al. Predictive validity of DSM‐IV and ICD‐10 criteria for ADHD and hyperkinetic disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines 2008;49(1):70‐8. - PubMed
Liberati 2009
Light 2015
Loney 1982
-
- Loney J, Milich R. Hyperactivity, inattention, and aggression in clinical practice. In: Wolraich M, Routh DK editor(s). Advances in Developmental and Behavioral Pediatrics. Vol. 3, Greenwich: JAI Press, 1982:113‐47.
Loney 1984
-
- Loney J. A short parent scale for subgrouping childhood hyperactivity, aggression. Annual Meeting of the American Psychological Association; 1984 August 24‐28; Toronto. Toronto, 1984.
Lundh 2012
Magnusson 2014a [pers comm]
-
- Magnusson FL. Received information on randomisation and allocation concealment procedure [personal communication]. Email to: Dr Klorman 17 April 2014.
Magnusson 2014b [pers comm]
-
- Magnusson FM. Data received from author [personal communication]. Email to: Dr Rapport 8 May 2014.
Magnusson 2014c [pers comm]
-
- Magnusson F. Data received from author [personal communication]. Email to: Dr Kircher May 2014.
Maia 2014
-
- Maia CR, Cortese S, Caye A, Deakin TK, Polanczyk GV, Polanczyk CA, et al. Long‐term efficacy of methylphenidate immediate‐release for the treatment of childhood ADHD: a systematic review and meta‐analysis. Journal of Attention Disorders 2010 December 14 [Epub ahead of print]. [DOI: 10.1177/1087054714559643] - DOI - PubMed
Marsee 2007
-
- Marsee MA, Frick PJ. Exploring the cognitive and emotional correlates to proactive and reactive aggression in a sample of detained girls. Journal of Abnormal Child Psychology 2007;35(6):969‐81. - PubMed
McGough 2006a
-
- McGough JJ, Wigal SB, Abikoff H, Turnbow JM, Posner K, Moon E. A randomized, double‐blind, placebo‐controlled, laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. Journal of Attention Disorders 2006;9(3):476‐85. - PubMed
Mendelsohn 1978
-
- Mendelsohn M, Erdwins C. The Disruptive Behavior Scale: an objective assessment of unmanageable social behavior in adolescents. Journal of Clinical Psychology 1978;34(2):426‐8. - PubMed
Milich 1980
-
- Milich R, Roberts MA, Loney J, Caputo J. Differentiating practice effects and statistical regression on the Conners Hyperkinesis Index. Journal of Abnormal Child Psychology 1980;8(4):549‐52. - PubMed
Miller 1997
-
- Miller LS, Koplewicz HS, Klein RG. Teacher ratings of hyperactivity, inattention, and conduct problems in preschoolers. Journal of Abnormal Child Psychology 1997;25(2):113‐9. - PubMed
Moffit 2007
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad A, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analysis?. Lancet 1998;352(9128):609‐13. - PubMed
Moher 2009
Moher 2010
Moher 2015
Moncrieff 2004
MTA 1999
-
- MTA Cooperation Group. A 14 month randomised clinical trial of treatment strategies for attention‐deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal treatment study of children with ADHD. Archives of General Psychiatry 1999;56(12):1073‐86. - PubMed
Murray 2007
-
- Murray DW, Kollins SH, Hardy KK, Abikoff HB, Swanson JM, Cunningham C, et al. Parent versus teacher ratings of attention‐deficit/hyperactivity disorder symptoms in the Preschoolers with Attention‐Deficit/Hyperactivity Disorder Treatment Study (PATS). Journal of Child and Adolescent Psychopharmacology 2007;17(5):605‐19. - PubMed
Murray 2009
Mustafa 2013
-
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42. - PubMed
NICE 2009
-
- National Collaborating Centre for Mental Health commissioned by the National Institute for Health and Clinical Excellence. Attention Deficit Hyperactivity Disorder. Diagnosis and Management of ADHD in Children, Young People and Adults. National Clinical Practice Guideline Number 72. Leicester and London: The British Psychological Society and The Royal College of Psychiatrists, 2009. - PubMed
Nigg 2005
-
- Nigg JT, Casey BJ. An integrative theory of attention‐deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology 2005;17(3):785‐806. - PubMed
Nilausen 2014 [pers comm]
-
- Nilausen TD. Requested data [personal communication]. Email to: Dr Kolko 13 January 2014.
Owens 2000
-
- Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school‐aged children. Sleep 2000;23(8):1043‐51. - PubMed
Pasini 2007
-
- Pasini A, Paloscia C, Alessandrelli R, Porfirio MC, Curatolo P. Attention and executive functions profile in drug naive ADHD subtypes. Brain & Development 2007;29(7):400‐8. - PubMed
Pelham 1993b
-
- Pelham WE Jr. Pharmacotherapy for children with attention‐deficit hyperactivity disorder. School Psychology Review 1993;22(2):199‐227.
Pelham 2005a
-
- Pelham WE, Burrows‐MacLean L, Gnagy EM, Fabiano GA, Coles EK, Tresco KE, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Experimental and Clinical Psychopharmacology 2005;13(2):111‐26. - PubMed
Pfefferbaum‐Levine 1983
-
- Pfefferbaum‐Levine B, Overall JE. Diagnostic factor structure of the Children's Psychiatric Rating Scale (C.P.R.S.). Journal of Clinical Child Psychology 1983;12(2):167‐73.
Pliszka 1998
-
- Pliszka S. The use of psychostimulants in the paediatric patient. Pediatric Clinics of North America 1998;45(5):1085‐98. - PubMed
Pliszka 2007a
-
- Pliszka S, AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(7):894‐921. - PubMed
Polanczyk 2007
-
- Polanczyk G, Rohde LA. Epidemiology of attention‐deficit/hyperactivity disorder across the lifespan. Current Opinion in Psychiatry 2007;20(4):386‐92. - PubMed
Polanczyk 2010
-
- Polanczyk G, Laranjeira R, Zalesk IM, Pinsky I, Caetano R, Rohde LA. ADHD in a representative sample of the Brazilian population: estimated prevalence and comparative adequacy of criteria between adolescents and adults according to the response theory. International Journal of Methods in Psychiatric Research 2010;19(3):177‐84. [DOI: 10.1002/mpr.319] - DOI - PMC - PubMed
Polanczyk 2014
Polderman 2007
-
- Polderman TJ, Derks EM, Hudziak JJ, Verhulst FC, Posthuma D, Boomsma DI. Across the continuum of attention skills: a twin study of the SWAN ADHD rating scale. Journal of Child Psychology and Psychiatry 2007;48(11):1080‐7. - PubMed
Poznanski 1983
-
- Poznanski EO, Cook SC, Carroll BJ, Corzo H. Use of the Children's Depression Rating Scale in an inpatient psychiatric population. Journal of Clinical Psychiatry 1983;44(6):200‐3. - PubMed
Požgain 2014
-
- Požgain I, Požgain Z, Degmečić D. Placebo and nocebo effect: a mini review. Psychiatria Danubina 2014;26(2):100‐7. - PubMed
Punja 2013
Raman 2015
-
- Raman SR, Marshall SW, Gaynes BN, Haynes K, Naftel AJ, Stürmer T. An observational study of pharmacological treatment in primary care of children with ADHD in the United Kingdom. Psychiatric Services 2015 March 1 [Epub ahead of print]. - PubMed
Ramstad 2013a [pers comm]
-
- Ramstad E. Data requested from author [personal communication]. Email to: Dr Ialongo 1 August 2013.
Ramstad 2013b [pers comm]
-
- Ramstad E. Request on data from the Rhode Island CLC study [personal communication]. Email to: Dr DuPaul 5 September 2013.
Ramstad 2013c [pers comm]
-
- Ramstad E. Data requestioned [personal communication]. Email to: Dr Rapport 22 July 2013.
Ramstad 2013d [pers comm]
-
- Ramstad E. Data requested from author [personal communication]. Email to: Dr Tirosh 1 August 2013.
Ramstad 2014 [pers comm]
-
- Ramstad E. Data received from authors [personal communication]. Email to: Dr Vitiello July 2014.
Reichow 2013
Rentz 2005
-
- Rentz AM, Matza LS, Secnik K, Swensen A, Revicki DA. Psychometric validation of the Child Health Questionnaire (CHQ) in a sample of children and adolescents with attention‐deficit/hyperactivity disorder. Quality of Life Research 2005;14(3):719‐34. - PubMed
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2004
-
- Riley AW, Forrest CB, Starfield B, Rebok GW, Robertson JA, Green BF. The Parent Report Form of the CHIP‐Child Edition: reliability and validity. Medical Care 2004;42(3):210‐20. - PubMed
Routh 1978
-
- Routh D. Hyperactivity. In: Magrab P editor(s). Psychological Management of Paediatric Problems. Baltimore: University Park Press, 1978.
Rowe 1997
-
- Rowe KS, Rowe KJ. Norms for parental ratings on Conners' Abbreviated Parent‐Teacher Questionnaire: implications for the design of behavioral rating inventories and analyses of data derived from them. Journal of Abnormal Child Psychology 1997;25(6):425‐51. - PubMed
Savović 2012a
-
- Savović J, Jones H, Altman D, Harris R, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429–38. - PubMed
Scahill 2000
-
- Scahill L, Schwab‐Stone M. Epidemiology of ADHD in school‐age children. Child and Adolescent Psychiatric Clinics of North America 2000;9(3):541‐55. - PubMed
Schachar 1997b
-
- Schachar RJ, Tannock R, Cunningham C, Corkum PV. Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(6):754‐63. - PubMed
Schachar 2007
Schachter 2001
Schmidt 2009
Schroll 2015
-
- Schroll J, Bero L. Editorial: regulatory agencies hold the key to improving Cochrane Reviews of drugs. bit.ly/1D6XyYc (accessed 6 July 2015). - PMC - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Schulz 2012
-
- Schulz KP, Fan J, Bédard AC, Clerkin SM, Ivanov I, Tang CY, et al. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention‐deficit/hyperactivity disorder. Archives of General Psychiatry 2012;69(9):952‐61. - PubMed
Sergeant 2003
-
- Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: a neuropsychological perspective. Neuroscience and Biobehavioural Reviews 2003;27(7):583‐92. - PubMed
Shaffer 1983
-
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A Children's Global Assessment Scale (CGAS). Archives of General Psychiatry 1983;40(11):1228‐31. - PubMed
Shaw 2012
SIGN 2009
-
- Scottish Intercollegiate Guidelines Network. Management of attention deficit and hyperkinetic disorders in children and young people. http://www.sign.ac.uk/guidelines/fulltext/112/ (accessed 30 April 2015).
Silva 2005b
-
- Silva RR, Alpert M, Pouget E, Silva V, Trosper S, Reyes K, et al. A rating scale for disruptive behavior disorders, based on the DSM‐IV item pool. Psychiatric Quarterly 2005;76(4):327‐39. [PUBMED: 16217627] - PubMed
Solanto 1998
-
- Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention‐deficit hyperactivity disorder: a review and integration. Behavioural Brain Research 1998;94(1):127‐52. - PubMed
Sonuga‐Barke 2007
-
- Sonuga‐Barke EJS, Coghill D, Markowitz JS, Swanson JM, Vandenberghe M, Hatch SJ. Sex differences in response of children with ADHD to once‐daily formulations of methylphenidate. Journal of American Academy of Child & Adolescent Psychiatry 2007;46(6):701‐10. - PubMed
Sprafkin 1986
-
- Sprafkin J, Grayson P, Gadow K, Nolan EE, Paolicelli LM. Code for Observing Social Activity (COSA). Stony Brook: State University of New York, Department of Psychiatry and Behavioral Science, 1986.
Steinhausen 1993
-
- Steinhausen H‐C. LF ‐ Teacher Questionnaire after Rutter, Tizard and Whitmore ‐ German edited version [Lehrerfragebogen nach Rutter, Tizard und Whitmore ‐ Deutsche bearbeitete Fassung]. Psychische Störungen bei Kindern und Jugendlichen. Lehrbuch der Kinder‐ und Jugendpsychiatrie. München: Urban Und Schwarzenberg, 1993.
Sterne 2013
Stevenson 1989
-
- Stevenson RD, Wolraich ML. Stimulant medication therapy in the treatment of children with attention deficit hyperactivity disorder. Pediatric Clinics of North America 1989;36(5):1183‐97. - PubMed
Storebø 2015b [pers comm]
-
- Storebø OJ, Gluud C. Criticism to "Immediate‐release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults" [personal communication]. Email to: T Epstein via Wiley Online Feedback form 10 May 2015.
Storebø in press
-
- Storebø OJ, Pedersen N, Krogh H, Ramstad E, Nilausen TD, Skoog M, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents ‐ assessment of harms in observational studies. Cochrane Database of Systematic Reviews in press.
Swanson 1992
-
- Swanson JM. School‐based Assessments and Interventions for ADD Students. Irvine, CA: KC Publishing, 1992.
Swanson 2004a
-
- Swanson JM, Wigal SB, Wigal T, Sonuga‐Barke E, Greenhill LL, Biederman J, et al. A comparison of once‐daily extended‐release methylphenidate formulations in children with attention‐deficit/hyperactivity disorder in the laboratory school (the COMACS Study). Pediatrics 2004;113(3):e206‐16. - PubMed
Swanson 2006
-
- Swanson JM, Schuck S, Mann M, Carlson C, Hartman K, Sergeant JA, et al. Categorical and dimensional definitions and evaluations of symptoms of ADHD: the SNAP and SWAN Rating Scales. http://bit.ly/1Kj9akF (accessed 4 March 2015). - PMC - PubMed
Swanson 2009
-
- Swanson J. The MTA follow‐up into adolescence: implications for personalized treatment. Oral presentation at The International Society for Research in Child and Adolescent Psychopathology (ISRCAP); 2009 June 19; Seattle. 2009.
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analysis?. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Timimi 2004
-
- Timimi S, Taylor E. ADHD is best understood as a cultural construct. British Journal of Psychiatry 2004;184(1):8‐9. - PubMed
Todd 2008
-
- Todd RD, Huang H, Henderson CA. Poor utility of the age of onset criterion for DSM‐IV attention deficit/hyperactivity disorder: recommendations for DSM‐V and ICD‐11. Journal of Child Psychology and Psychiatry and Allied Disciplines 2008;49(9):942‐9. - PubMed
Torrance 1982
-
- Torrance GW, Boyle MH, Horwood SP. Application of multi‐attribute utility theory to measure social preferences for health states. Operations Research 1982;30(6):1043‐69. - PubMed
Tripp 1999
-
- Tripp G, Luk SL, Schaughency EA, Singh R. DSM‐IV and ICD‐10: a comparison of the correlates of ADHD and hyperkinetic disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(2):156‐64. - PubMed
Turgay 1994
-
- Turgay A. Disruptive Behavior Disorders: Child and Adolescent Screening and Rating Scales for Children, Adolescents, Parents and Teachers. West Blomfield, Michigan: Integrative Therapy Institute Publication, 1994.
Ullmann 1984
-
- Ullmann RK, Sleator EK, Sprague RL. A new rating scale for diagnosis and monitoring of ADD children. Psychopharmacology Bulletin 1984;20(1):160‐4. - PubMed
US FDA 2011
-
- US Food, Drug Administration. Communication about an ongoing safety review of stimulant medications used in children with attention‐deficit/hyperactivity disorder (ADHD). http://1.usa.gov/1M5AYoq (accessed 6 May 2011).
Van der Meere 1999b
-
- Meere J, Gunning B, Stemerdink N. The effect of methylphenidate and clonidine on response inhibition and state regulation in children with ADHD. Journal of Child Psychology and Psychiatry and Allied Disciplines 1999;40(2):291‐8. - PubMed
Van der Oord 2008
-
- Oord S, Prins PJM, Oosterlaan J, Emmelkamp PMG. Efficacy of methylphenidate, psychosocial treatments and their combination in school‐aged children with ADHD: a meta‐analysis. Clinical Psychology Review 2008;28(5):783‐800. - PubMed
Visser 2014
-
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, et al. Trends in the parent‐report of health care provider‐diagnosed and medicated attention‐deficit/hyperactivity disorder: United States, 2003‐2011. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(1):34‐46.e2. - PMC - PubMed
Vitiello 2008
Wahler 1976
-
- Wahler RG, House AE, Stambaugh EE. Ecological Assessment of Child Problem Behavior: A Clinical Package for Home, School, and Institutional Settings. Oxford: Pergamon, 1976.
Ward 1993
-
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. American Journal of Psychiatry 1993;150(6):885‐90. - PubMed
Wechsler 1945
-
- Wechsler D. A standardized memory scale for clinical use. Journal of Psychology: Interdisciplinary and Applied 1945;19(1):87‐95.
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
WHO 1992
-
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders. Clinical Descriptions and Diagnostic Guidelines. Geneva: WHO, 1992.
Wigal 1998
-
- Wigal SB, Gupta S, Guinta D, Swanson JM. Reliability and validity of the SKAMP rating scale in a laboratory school setting. Psychopharmacology Bulletin 1998;34(1):47‐53. - PubMed
Wilens 2006a
-
- Wilens TE, Gignac M, Swezey A, Monuteaux MC, Biederman J. Characteristics of adolescents and young adults with ADHD who divert or misuse their prescribed medications. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(4):408‐14. - PubMed
Wilkinson 2006
-
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4. Lutz, FL: Psychological Assessment Resources, 2006.
Willcut 2012
Wolraich 2003
-
- Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. Journal of Pediatric Psychology 2003;28(8):559‐67. - PubMed
Wood 2008
Woodcock 2001
-
- Woodcock RW, Mcgrew KS, Mather N. Woodcock‐Johnson III Tests of Achievement. Itasca, IL: Riverside Publishers, 2001.
Young 1978
-
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry 1978;133(11):429‐35. - PubMed
Zhang 2005
Zito 2000
-
- Zito J, Safer DJ, DosReis S, Gardner JF, Boles M, Lynch FT. Trends in the prescribing of psychotropic medications to preschoolers. JAMA 2000;283(8):1025‐30. - PubMed
References to other published versions of this review
Storebø 2012
-
- Storebø OJ, Rosendal S, Skoog M, Groth C, Bille T, Buch Rasmussen K, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009885] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

