Development of an Aquaporin-4 Orthogonal Array of Particle-Based ELISA for Neuromyelitis Optica Autoantibodies Detection
- PMID: 26599905
- PMCID: PMC4658006
- DOI: 10.1371/journal.pone.0143679
Development of an Aquaporin-4 Orthogonal Array of Particle-Based ELISA for Neuromyelitis Optica Autoantibodies Detection
Abstract
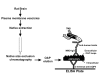
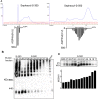
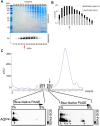
Serological markers of Nuromyelitis Optica (NMO), an autoimmune disorder of the central nervous system, are autoantibodies targeting the astrocytic water channel aquaporin-4 (AQP4). We have previously demonstrated that the main epitopes for these autoantibodies (AQP4-IgG) are generated by the supramolecular arrangement of AQP4 tetramers into an Orthogonal Array of Particles (OAPs). Many tests have been developed to detect AQP4-IgG in patient sera but several procedural issues affect OAP assembly and consequently test sensitivity. To date, the protein based ELISA test shows the lowest sensitivity while representing a valid alternative to the more sensitive cell based assay (CBA), which, however, shows economic, technical and interpretation problems. Here we have developed a high perfomance ELISA in which native OAPs are used as the molecular target. To this aim a native size exclusion chromatography method has been developed to isolate integral, highly pure and AQP4-IgG-recognized OAPs from rat brain. These OAPs were immobilized and oriented on a plastic plate by a sandwich approach and 139 human sera were tested, including 67 sera from NMO patients. The OAP-ELISA showed a 99% specificity and a higher sensitivity (91%) compared to the CBA test. A comparative analysis revealed an end-point titer three orders of magnitude higher than the commercial ELISA and six times higher than our in-house CBA test. We show that CNS-extracted OAPs are crucial elements in order to perform an efficient AQP4-IgG test and the OAP-ELISA developed represents a valid alternative to the CBA currently used.
Conflict of interest statement
Figures




References
-
- Bergamaschi R, Ghezzi A. Devic's neuromyelitis optica: clinical features and prognostic factors. Neurol Sci. 2004;25 Suppl 4:S364–7. - PubMed
-
- Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69(24):2221–31. - PubMed
-
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

