High-Throughput Screening of Myometrial Calcium-Mobilization to Identify Modulators of Uterine Contractility
- PMID: 26600013
- PMCID: PMC4658040
- DOI: 10.1371/journal.pone.0143243
High-Throughput Screening of Myometrial Calcium-Mobilization to Identify Modulators of Uterine Contractility
Abstract
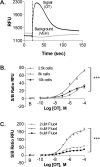
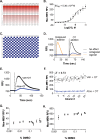
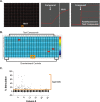
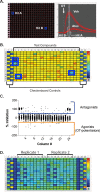
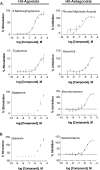
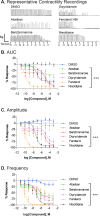
The uterine myometrium (UT-myo) is a therapeutic target for preterm labor, labor induction, and postpartum hemorrhage. Stimulation of intracellular Ca2+-release in UT-myo cells by oxytocin is a final pathway controlling myometrial contractions. The goal of this study was to develop a dual-addition assay for high-throughput screening of small molecular compounds, which could regulate Ca2+-mobilization in UT-myo cells, and hence, myometrial contractions. Primary murine UT-myo cells in 384-well plates were loaded with a Ca2+-sensitive fluorescent probe, and then screened for inducers of Ca2+-mobilization and inhibitors of oxytocin-induced Ca2+-mobilization. The assay exhibited robust screening statistics (Z´ = 0.73), DMSO-tolerance, and was validated for high-throughput screening against 2,727 small molecules from the Spectrum, NIH Clinical I and II collections of well-annotated compounds. The screen revealed a hit-rate of 1.80% for agonist and 1.39% for antagonist compounds. Concentration-dependent responses of hit-compounds demonstrated an EC50 less than 10μM for 21 hit-antagonist compounds, compared to only 7 hit-agonist compounds. Subsequent studies focused on hit-antagonist compounds. Based on the percent inhibition and functional annotation analyses, we selected 4 confirmed hit-antagonist compounds (benzbromarone, dipyridamole, fenoterol hydrobromide and nisoldipine) for further analysis. Using an ex vivo isometric contractility assay, each compound significantly inhibited uterine contractility, at different potencies (IC50). Overall, these results demonstrate for the first time that high-throughput small-molecules screening of myometrial Ca2+-mobilization is an ideal primary approach for discovering modulators of uterine contractility.
Conflict of interest statement
Figures

Similar articles
-
Comparative analysis of myometrial and vascular smooth muscle cells to determine optimal cells for use in drug discovery.Pharmacol Res. 2019 Aug;146:104268. doi: 10.1016/j.phrs.2019.104268. Epub 2019 May 9. Pharmacol Res. 2019. PMID: 31078743 Free PMC article.
-
The role of cyclic-ADP-ribose-signaling pathway in oxytocin-induced Ca2+ transients in human myometrium cells.Endocrinology. 2004 Feb;145(2):881-9. doi: 10.1210/en.2003-0774. Epub 2003 Oct 16. Endocrinology. 2004. PMID: 14563702
-
Effects of calcium channel blocker, mibefradil, and potassium channel opener, pinacidil, on the contractile response of mid-pregnant goat myometrium.Indian J Exp Biol. 2005 Sep;43(9):795-801. Indian J Exp Biol. 2005. PMID: 16187530
-
Oxytocin and parturition: a role for increased myometrial calcium and calcium sensitization?Front Biosci. 2007 Jan 1;12:619-33. doi: 10.2741/2087. Front Biosci. 2007. PMID: 17127323 Review.
-
Multiple mechanisms involved in oxytocin-induced modulation of myometrial contractility.Acta Pharmacol Sin. 2006 Jul;27(7):827-32. doi: 10.1111/j.1745-7254.2006.00393.x. Acta Pharmacol Sin. 2006. PMID: 16787565 Review.
Cited by
-
Transcription Factor 23 is an Essential Determinant of Murine Term Parturition.Mol Cell Biol. 2024;44(8):316-333. doi: 10.1080/10985549.2024.2376146. Epub 2024 Jul 16. Mol Cell Biol. 2024. PMID: 39014976 Free PMC article.
-
Drug discovery strategies for the identification of novel regulators of uterine contractility.Curr Opin Physiol. 2020 Feb;13:71-86. doi: 10.1016/j.cophys.2019.10.012. Epub 2019 Oct 23. Curr Opin Physiol. 2020. PMID: 32864532 Free PMC article.
-
Regulation of oxytocin-induced calcium transients and gene expression in engineered myometrial tissues by tissue architecture and matrix rigidity.Curr Res Physiol. 2023 Sep 21;6:100108. doi: 10.1016/j.crphys.2023.100108. eCollection 2023. Curr Res Physiol. 2023. PMID: 38107790 Free PMC article.
-
Phenylephrine, a common cold remedy active ingredient, suppresses uterine contractions through cAMP signalling.Sci Rep. 2018 Aug 3;8(1):11666. doi: 10.1038/s41598-018-30094-5. Sci Rep. 2018. PMID: 30076382 Free PMC article.
-
Effect of molecular hydrogen on uterine inflammation during preterm labour.Biomed Rep. 2018 May;8(5):454-460. doi: 10.3892/br.2018.1082. Epub 2018 Mar 27. Biomed Rep. 2018. PMID: 29732148 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

