Fibrogenic Lung Injury Induces Non-Cell-Autonomous Fibroblast Invasion
- PMID: 26600305
- PMCID: PMC4942213
- DOI: 10.1165/rcmb.2015-0040OC
Fibrogenic Lung Injury Induces Non-Cell-Autonomous Fibroblast Invasion
Abstract
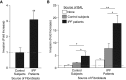
Pathologic accumulation of fibroblasts in pulmonary fibrosis appears to depend on their invasion through basement membranes and extracellular matrices. Fibroblasts from the fibrotic lungs of patients with idiopathic pulmonary fibrosis (IPF) have been demonstrated to acquire a phenotype characterized by increased cell-autonomous invasion. Here, we investigated whether fibroblast invasion is further stimulated by soluble mediators induced by lung injury. We found that bronchoalveolar lavage fluids from bleomycin-challenged mice or patients with IPF contain mediators that dramatically increase the matrix invasion of primary lung fibroblasts. Further characterization of this non-cell-autonomous fibroblast invasion suggested that the mediators driving this process are produced locally after lung injury and are preferentially produced by fibrogenic (e.g., bleomycin-induced) rather than nonfibrogenic (e.g., LPS-induced) lung injury. Comparison of invasion and migration induced by a series of fibroblast-active mediators indicated that these two forms of fibroblast movement are directed by distinct sets of stimuli. Finally, knockdown of multiple different membrane receptors, including platelet-derived growth factor receptor-β, lysophosphatidic acid 1, epidermal growth factor receptor, and fibroblast growth factor receptor 2, mitigated the non-cell-autonomous fibroblast invasion induced by bronchoalveolar lavage from bleomycin-injured mice, suggesting that multiple different mediators drive fibroblast invasion in pulmonary fibrosis. The magnitude of this mediator-driven fibroblast invasion suggests that its inhibition could be a novel therapeutic strategy for pulmonary fibrosis. Further elaboration of the molecular mechanisms that drive non-cell-autonomous fibroblast invasion consequently may provide a rich set of novel drug targets for the treatment of IPF and other fibrotic lung diseases.
Keywords: fibroblast; idiopathic pulmonary fibrosis; invasion; migration; pathogenesis.
Figures





References
-
- Selman M, King TE, Pardo A American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. - PubMed
-
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

