Imaging Diagnosis of Splanchnic Venous Thrombosis
- PMID: 26600801
- PMCID: PMC4620257
- DOI: 10.1155/2015/101029
Imaging Diagnosis of Splanchnic Venous Thrombosis
Abstract
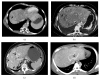
Splanchnic vein thrombosis (SVT) is a broad term that includes Budd-Chiari syndrome and occlusion of veins that constitute the portal venous system. Due to the common risk factors involved in the pathogenesis of these clinically distinct disorders, concurrent involvement of two different regions is quite common. In acute and subacute SVT, the symptoms may overlap with a variety of other abdominal emergencies while in chronic SVT, the extent of portal hypertension and its attendant complications determine the clinical course. As a result, clinical diagnosis is often difficult and is frequently reliant on imaging. Tremendous improvements in vascular imaging in recent years have ensured that this once rare entity is being increasingly detected. Treatment of acute SVT requires immediate anticoagulation. Transcatheter thrombolysis or transjugular intrahepatic portosystemic shunt is used in the event of clinical deterioration. In cases with peritonitis, immediate laparotomy and bowel resection may be required for irreversible bowel ischemia. In chronic SVT, the underlying cause should be identified and treated. The imaging manifestations of the clinical syndromes resulting from SVT are comprehensively discussed here along with a brief review of the relevant clinical features and therapeutic approach.
Figures

























































References
-
- Valla D. Hepatic venous outflow tract obstruction etipathogenesis: Asia versus the West. Journal of Gastroenterology and Hepatology. 2004;19, supplement:204–211.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

