Promising biological therapies for ulcerative colitis: A review of the literature
- PMID: 26600980
- PMCID: PMC4644886
- DOI: 10.4291/wjgp.v6.i4.219
Promising biological therapies for ulcerative colitis: A review of the literature
Abstract
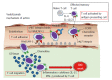
Ulcerative colitis (UC) is a chronic lifelong condition characterized by alternating flare-ups and remission. There is no single known unifying cause, and the pathogenesis is multifactorial, with genetics, environmental factors, microbiota, and the immune system all playing roles. Current treatment modalities for UC include 5-aminosalicylates, corticosteroids, immunosuppressants (including purine antimetabolites, cyclosporine, and tacrolimus), and surgery. Therapeutic goals for UC are evolving. Medical treatment aims to induce remission and prevent relapse of disease activity. Infliximab, an anti-tumor necrosis factor (TNF)-α monoclonal antibody, is the first biological agent for the treatment of UC. Over the last decade, infliximab and adalimumab (anti-TNF-α agents) have been used for moderate to severe UC, and have been shown to be effective in inducing and maintaining remission. Recent studies have indicated that golimumab (another anti-TNF-α agent), tofacitinib (a Janus kinase inhibitor), and vedolizumab and etrolizumab (integrin antagonists), achieved good clinical remission and response rates in UC. Recently, golimumab and vedolizumab have been approved for UC by the United States Food and Drug Administration. Vedolizumab may be used as a first-line alternative to anti-TNF-α therapy in patients with an inadequate response to corticosteroids and/or immunosuppressants. Here, we provide updated information on various biological agents in the treatment of UC.
Keywords: Anti-integrin agents; Anti-tumor necrosis factor α agents; Biological therapy; Janus kinase inhibitor; Ulcerative colitis.
Figures
References
-
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. - PubMed
-
- Engel MA, Neurath MF. New pathophysiological insights and modern treatment of IBD. J Gastroenterol. 2010;45:571–583. - PubMed
-
- Hanauer SB, Kirsner JB. Treat the patient or treat the disease? Dig Dis. 2012;30:400–403. - PubMed
-
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

