All-plastic, miniature, digital fluorescence microscope for three part white blood cell differential measurements at the point of care
- PMID: 26601006
- PMCID: PMC4646550
- DOI: 10.1364/BOE.6.004433
All-plastic, miniature, digital fluorescence microscope for three part white blood cell differential measurements at the point of care
Abstract
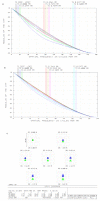
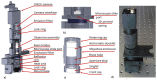
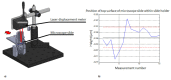
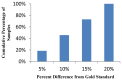
Three-part differential white blood cell counts are used for disease diagnosis and monitoring at the point-of-care. A low-cost, miniature achromatic microscope was fabricated for identification of lymphocytes, monocytes, and granulocytes in samples of whole blood stained with acridine orange. The microscope was manufactured using rapid prototyping techniques of diamond turning and 3D printing and is intended for use at the point-of-care in low-resource settings. The custom-designed microscope requires no manual adjustment between samples and was successfully able to classify three white blood cell types (lymphocytes, granulocytes, and monocytes) using samples of peripheral whole blood stained with acridine orange.
Keywords: (170.1470) Blood or tissue constituent monitoring; (170.2520) Fluorescence microscopy; (170.3880) Medical and biological imaging.
Figures




References
-
- Blumenreich M. S., Clinical Methods: The History, Physical, and Laboratory Examinations (Butterworths, 1990). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
