Co-infections determine patterns of mortality in a population exposed to parasite infection
- PMID: 26601143
- PMCID: PMC4643819
- DOI: 10.1126/sciadv.1400026
Co-infections determine patterns of mortality in a population exposed to parasite infection
Abstract
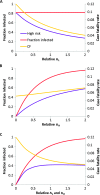
Many individual hosts are infected with multiple parasite species, and this may increase or decrease the pathogenicity of the infections. This phenomenon is termed heterologous reactivity and is potentially an important determinant of both patterns of morbidity and mortality and of the impact of disease control measures at the population level. Using infections with Theileria parva (a tick-borne protozoan, related to Plasmodium) in indigenous African cattle [where it causes East Coast fever (ECF)] as a model system, we obtain the first quantitative estimate of the effects of heterologous reactivity for any parasitic disease. In individual calves, concurrent co-infection with less pathogenic species of Theileria resulted in an 89% reduction in mortality associated with T. parva infection. Across our study population, this corresponds to a net reduction in mortality due to ECF of greater than 40%. Using a mathematical model, we demonstrate that this degree of heterologous protection provides a unifying explanation for apparently disparate epidemiological patterns: variable disease-induced mortality rates, age-mortality profiles, weak correlations between the incidence of infection and disease (known as endemic stability), and poor efficacy of interventions that reduce exposure to multiple parasite species. These findings can be generalized to many other infectious diseases, including human malaria, and illustrate how co-infections can play a key role in determining population-level patterns of morbidity and mortality due to parasite infections.
Keywords: East Coast fever; Epidemiology; Mathematical model; Theileria parva; case fatality; cattle; endemic stability; heterologous protection; malaria; vaccination.
Figures
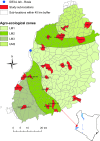
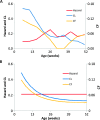
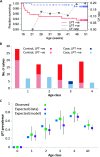
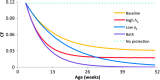
References
-
- Dye C., Williams B. G., The population dynamics and control of tuberculosis. Science 328, 856–861 (2010). - PubMed
-
- Read A. F., Taylor L. H., The ecology of genetically diverse infections. Science 292, 1099–1102 (2001). - PubMed
-
- Lello J., Boag B., Fenton A., Stevenson I. R., Hudson P. J., Competition and mutualism among the gut helminths of a mammalian host. Nature 428, 840–844 (2004). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

