Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase
- PMID: 26601215
- PMCID: PMC4646778
- DOI: 10.1126/sciadv.1500112
Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase
Abstract
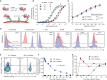
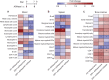
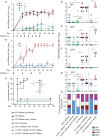
Antigen-specific immune responses to protein drugs can hinder efficacy and compromise safety because of drug neutralization and secondary clinical complications. We report a tolerance induction strategy to prevent antigen-specific humoral immune responses to therapeutic proteins. Our modular, biomolecular approach involves engineering tolerizing variants of proteins such that they bind erythrocytes in vivo upon injection, on the basis of the premise that aged erythrocytes and the payloads they carry are cleared tolerogenically, driving the deletion of antigen-specific T cells. We demonstrate that binding the clinical therapeutic enzyme Escherichia coli l-asparaginase to erythrocytes in situ antigen-specifically abrogates development of antibody titers by >1000-fold and extends the pharmacodynamic effect of the drug 10-fold in mice. Additionally, a single pretreatment dose of erythrocyte-binding asparaginase tolerized mice to multiple subsequent doses of the wild-type enzyme. This strategy for reducing antigen-specific humoral responses may enable more effective and safer treatment with therapeutic proteins and drug candidates that are hampered by immunogenicity.
Keywords: Immunological tolerance; asparaginase; erythrocyte; protein drug.
Figures

References
-
- Nechansky A., Kircheis R., Immunogenicity of therapeutics: A matter of efficacy and safety. Expert Opin. Drug Discov. 5, 1067–1079 (2010). - PubMed
-
- Joly M. S., Martin R. P., Mitra-Kaushik S., Phillips L., D’Angona A., Richards S. M., Joseph A. M., Transient low-dose methotrexate generates B regulatory cells that mediate antigen-specific tolerance to alglucosidase α. J. Immunol. 193, 3947–3958 (2014). - PubMed
-
- Banugaria S. G., Prater S. N., McGann J. K., Feldman J. D., Tannenbaum J. A., Bailey C., Gera R., Conway R. L., Viskochil D., Kobori J. A., Rosenberg A. S., Kishnani P. S., Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: Lessons learned from Pompe disease. Genet. Med. 15, 123–131 (2013). - PMC - PubMed
-
- Garay R. P., El-Gewely R., Armstrong J. K., Garratty G., Richette P., Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 9, 1319–1323 (2012). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

