Hierarchical Ni-Mo-S nanosheets on carbon fiber cloth: A flexible electrode for efficient hydrogen generation in neutral electrolyte
- PMID: 26601227
- PMCID: PMC4643797
- DOI: 10.1126/sciadv.1500259
Hierarchical Ni-Mo-S nanosheets on carbon fiber cloth: A flexible electrode for efficient hydrogen generation in neutral electrolyte
Abstract
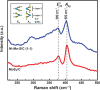
A unique functional electrode made of hierarchal Ni-Mo-S nanosheets with abundant exposed edges anchored on conductive and flexible carbon fiber cloth, referred to as Ni-Mo-S/C, has been developed through a facile biomolecule-assisted hydrothermal method. The incorporation of Ni atoms in Mo-S plays a crucial role in tuning its intrinsic catalytic property by creating substantial defect sites as well as modifying the morphology of Ni-Mo-S network at atomic scale, resulting in an impressive enhancement in the catalytic activity. The Ni-Mo-S/C electrode exhibits a large cathodic current and a low onset potential for hydrogen evolution reaction in neutral electrolyte (pH ~7), for example, current density of 10 mA/cm(2) at a very small overpotential of 200 mV. Furthermore, the Ni-Mo-S/C electrode has excellent electrocatalytic stability over an extended period, much better than those of MoS2/C and Pt plate electrodes. Scanning and transmission electron microscopy, Raman spectroscopy, x-ray diffraction, x-ray photoelectron spectroscopy, and x-ray absorption spectroscopy were used to understand the formation process and electrocatalytic properties of Ni-Mo-S/C. The intuitive comparison test was designed to reveal the superior gas-evolving profile of Ni-Mo-S/C over that of MoS2/C, and a laboratory-scale hydrogen generator was further assembled to demonstrate its potential application in practical appliances.
Keywords: Carbon Fiber Cloth; Flexible Electrodes; Hierarchical Ni-Mo-S Nanosheets; Hydrogen Generation; Neutral Electrolyte.
Figures








References
-
- Walter M. G., Warren E. L., McKone J. R., Boettcher S. W., Mi Q., Santori E. A., Lewis N. S., Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010). - PubMed
-
- A. Lasia, Hydrogen evolution reaction, in Handbook of Fuel Cells: Fundamentals, Technology and Applications (Wiley, New York, NY, 2010), vol. 2, pp. 416–440.
-
- Hinnemann B., Moses P. G., Bonde J., Jørgensen K. P., Nielsen J. H., Horch S., Chorkendorff I., Nørskov J. K., Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 127, 5308–5309 (2005). - PubMed
-
- Conway B. E., Tilak B. V., Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 47, 3571–3594 (2002).
-
- Huang X., Zeng Z., Zhang H., Metal dichalcogenide nanosheets: Preparation, properties and applications. Chem. Soc. Rev. 42, 1934–1946 (2013). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

