Spectrometer-free vibrational imaging by retrieving stimulated Raman signal from highly scattered photons
- PMID: 26601311
- PMCID: PMC4646825
- DOI: 10.1126/sciadv.1500738
Spectrometer-free vibrational imaging by retrieving stimulated Raman signal from highly scattered photons
Abstract
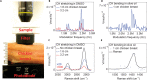
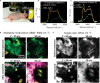
In vivo vibrational spectroscopic imaging is inhibited by relatively slow spectral acquisition on the second scale and low photon collection efficiency for a highly scattering system. Recently developed multiplex coherent anti-Stokes Raman scattering and stimulated Raman scattering techniques have improved the spectral acquisition time down to microsecond scale. These methods using a spectrometer setting are not suitable for turbid systems in which nearly all photons are scattered. We demonstrate vibrational imaging by spatial frequency multiplexing of incident photons and single photodiode detection of a stimulated Raman spectrum within 60 μs. Compared to the spectrometer setting, our method improved the photon collection efficiency by two orders of magnitude for highly scattering specimens. We demonstrated in vivo imaging of vitamin E distribution on mouse skin and in situ imaging of human breast cancerous tissues. The reported work opens new opportunities for spectroscopic imaging in a surgical room and for development of deep-tissue Raman spectroscopy toward molecular level diagnosis.
Keywords: Raman spectroscopy; label-free microscopy; molecular vibration.
Figures


References
-
- Hong S., Chen T., Zhu Y., Li A., Huang Y., Chen X., Live-cell stimulated Raman scattering imaging of alkyne-tagged biomolecules. Angew. Chem. Int. Ed. 53, 5827–5831 (2014). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

