Safety and efficacy of re-treatments with pyronaridine-artesunate in African patients with malaria: a substudy of the WANECAM randomised trial
- PMID: 26601738
- PMCID: PMC4726763
- DOI: 10.1016/S1473-3099(15)00318-7
Safety and efficacy of re-treatments with pyronaridine-artesunate in African patients with malaria: a substudy of the WANECAM randomised trial
Abstract
Background: Sparse data on the safety of pyronaridine-artesunate after repeated treatment of malaria episodes restrict its clinical use. We therefore compared the safety of pyronaridine-artesunate after treatment of the first episode of malaria versus re-treatment in a substudy analysis.
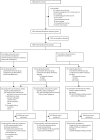
Methods: This planned substudy analysis of the randomised, open-label West African Network for Clinical Trials of Antimalarial Drugs (WANECAM) phase 3b/4 trial was done at six health facilities in Mali, Burkina Faso, and Guinea in patients (aged ≥6 months and bodyweight ≥5 kg) with uncomplicated microscopically confirmed Plasmodium spp malaria (parasite density <200 000 per μL blood) and fever or history of fever. The primary safety endpoint was incidence of hepatotoxicity: alanine aminotransferase of greater than five times the upper limit of normal (ULN) or Hy's criteria (alanine aminotransferase or aspartate aminotransferase greater than three times the ULN and total bilirubin more than twice the ULN) after treatment of the first episode of malaria and re-treatment (≥28 days after first treatment) with pyronaridine-artesunate. Pyronaridine-artesunate efficacy was compared with artemether-lumefantrine with the adequate clinical and parasitological response (ACPR) in an intention-to-treat analysis. WANECAM is registered with PACTR.org, number PACTR201105000286876.
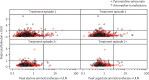
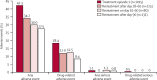
Findings: Following first treatment, 13 (1%) of 996 patients had hepatotoxicity (including one [<1%] possible Hy's law case) versus two (1%) of 311 patients on re-treatment (neither a Hy's law case). No evidence was found that pyronaridine-artesunate re-treatment increased safety risk based on laboratory values, reported adverse event frequencies, or electrocardiograph findings. For all first treatment or re-treatment episodes, pyronaridine-artesunate (n=673) day 28 crude ACPR was 92·7% (95% CI 91·0-94·3) versus 80·4% (77·8-83·0) for artemether-lumefantrine (n=671). After exclusion of patients with PCR-confirmed new infections, ACPR was similar on treatment and re-treatment and greater than 95% at day 28 and greater than 91% at day 42 in both treatment groups.
Interpretation: The findings that pyronaridine-artesunate safety and efficacy were similar on first malaria treatment versus re-treatment of subsequent episodes lend support for the wider access to pyronaridine-artesunate as an alternative artemisinin-based combination treatment for malaria in sub-Saharan Africa.
Funding: European and Developing Countries Clinical Trial Partnership, Medicines for Malaria Venture (Geneva, Switzerland), UK Medical Research Council, Swedish International Development Cooperation Agency, German Ministry for Education and Research, University Claude Bernard (Lyon, France), Malaria Research and Training Centre (Bamako, Mali), Centre National de Recherche et de Formation sur le Paludisme (Burkina Faso), Institut de Recherche en Sciences de la Santé (Bobo-Dioulasso, Burkina Faso), and Centre National de Formation et de Recherche en Santé Rurale (Republic of Guinea).
Copyright © 2016 Sagara et al. Open Access article distributed under the terms of CC BY-NC-ND. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Pyronaridine-artesunate retreatment for malaria.Lancet Infect Dis. 2016 Feb;16(2):136-7. doi: 10.1016/S1473-3099(15)00353-9. Epub 2015 Oct 23. Lancet Infect Dis. 2016. PMID: 26601737 No abstract available.
References
-
- Committee for Medicinal Products for Human Use (CHMP) Summary of opinion: Pyramax (pyronaridine tetraphosphate/artesunate) 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2012/02/WC500... (accessed Sept 8, 2014).
-
- Rueangweerayut R, Phyo AP, Uthaisin C. Pyronaridine-artesunate versus mefloquine plus artesunate for malaria. N Engl J Med. 2012;366:1298–1309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

