Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria
- PMID: 26601902
- PMCID: PMC4917673
- DOI: 10.1038/mi.2015.121
Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria
Abstract
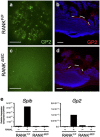
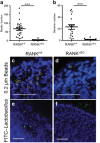
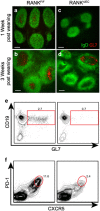
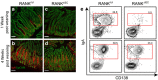
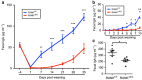
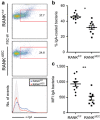
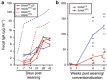
Secretory IgA (SIgA) directed against gut resident bacteria enables the mammalian mucosal immune system to establish homeostasis with the commensal gut microbiota after weaning. Germinal centers (GCs) in Peyer's patches (PPs) are the principal inductive sites where naive B cells specific for bacterial antigens encounter their cognate antigens and receive T-cell help driving their differentiation into IgA-producing plasma cells. We investigated the role of antigen sampling by intestinal M cells in initiating the SIgA response to gut bacteria by developing mice in which receptor activator of nuclear factor-κB ligand (RANKL)-dependent M-cell differentiation was abrogated by conditional deletion of Tnfrsf11a in the intestinal epithelium. Mice without intestinal M cells had profound delays in PP GC maturation and emergence of lamina propria IgA plasma cells, resulting in diminished levels of fecal SIgA that persisted into adulthood. We conclude that M-cell-mediated sampling of commensal bacteria is a required initial step for the efficient induction of intestinal SIgA.
Figures
References
-
- Macpherson, A.J., McCoy, K.D., Johansen, F.E. & Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

